Toll-like Receptor Agonist Conjugation: A Chemical Perspective
- PMID: 29378134
- PMCID: PMC10642707
- DOI: 10.1021/acs.bioconjchem.7b00808
Toll-like Receptor Agonist Conjugation: A Chemical Perspective
Abstract
Toll-like receptors (TLRs) are vital elements of the mammalian immune system that function by recognizing pathogen-associated molecular patterns (PAMPs), bridging innate and adaptive immunity. They have become a prominent therapeutic target for the treatment of infectious diseases, cancer, and allergies, with many TLR agonists currently in clinical trials or approved as immunostimulants. Numerous studies have shown that conjugation of TLR agonists to other molecules can beneficially influence their potency, toxicity, pharmacokinetics, or function. The functional properties of TLR agonist conjugates, however, are highly dependent on the ligation strategy employed. Here, we review the chemical structural requirements for effective functional TLR agonist conjugation. In addition, we provide similar analysis for those that have yet to be conjugated. Moreover, we discuss applications of covalent TLR agonist conjugation and their implications for clinical use.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

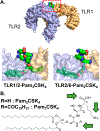
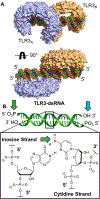

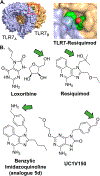
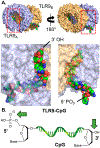
References
-
- Kawai T, and Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373–384. - PubMed
-
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, and Persing D (2004) Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin. Biol. Ther 4, 1129–1138. - PubMed
-
- Schön MP, and Schön M (2008) TLR7 and TLR8 as targets in cancer therapy. Oncogene 27, 190–199. - PubMed
-
- Kanzler H, Barrat FJ, Hessel EM, and Coffman RL (2007) Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med 13, 552–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

