Comparative Histopathologic Lesions of the Male Reproductive Tract during Acute Infection of Zika Virus in AG129 and Ifnar-/- Mice
- PMID: 29378173
- PMCID: PMC5955007
- DOI: 10.1016/j.ajpath.2017.12.019
Comparative Histopathologic Lesions of the Male Reproductive Tract during Acute Infection of Zika Virus in AG129 and Ifnar-/- Mice
Abstract
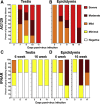
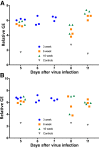
An understanding of the pathogenesis of infection with the Zika virus in the male reproductive tract is vital for the development of vaccines and antivirals that will limit or prevent sexual transmission. Two common immunocompromised mouse strains used in transmission studies-male with genes encoding interferon types I and II receptor gene knockout (IFNAR/IFNGR; AG129) and with interferon type 1 receptor knockout (Ifnar-/-) were infected with a Puerto Rican Zika virus isolate (PRVABC59), and pathology was assessed 5 to 11 days after infection. Virus was detected by immunohistochemistry and quantitative RT-PCR in the testicle and epididymis of AG129 and Ifnar-/- mice, and by immunohistochemistry in the prostate and seminal vesicle of infected AG129 mice. Severe disease manifestations initiating as epididymitis and progressing to orchitis were observed in both models, with more severe inflammation noted in the AG129 mouse strain. Significant inflammation was not observed in any evaluated accessory sex gland at any point during infection. Time-course analysis of infection revealed an increase in the severity of disease within the epididymis of both strains, indicating a potential route of sexual transmission. Male mice with Ifnar-/- may better recapitulate Zika virus in humans and provide insight into the mechanism of sexual transmission, due to milder histopathologic lesions, the presence of histologically normal sperm in epididymal tubules, and an ability to survive the acute phase of disease.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Dick G.W. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. - PubMed
-
- Roth A., Mercier A., Lepers C., Hoy D., Duituturaga S., Benyon E., Guillaumot L., Souarès Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. 2014;19 pii:20929. - PubMed
-
- Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., Dubray C., Guillaumot L., Griggs A., Bel M., Lambert A.J., Laven J., Kosoy O., Panella A., Biggerstaff B.J., Fischer M., Hayes E.B. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

