Activation of neural stem cells from quiescence drives reactive hippocampal neurogenesis after alcohol dependence
- PMID: 29378214
- PMCID: PMC6620048
- DOI: 10.1016/j.neuropharm.2018.01.032
Activation of neural stem cells from quiescence drives reactive hippocampal neurogenesis after alcohol dependence
Abstract
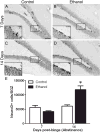
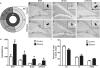
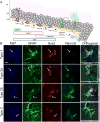
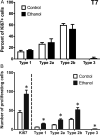
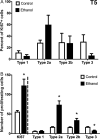
Neural stem cell-driven adult neurogenesis contributes to the integrity of the hippocampus. Excessive alcohol consumption in alcoholism results in hippocampal degeneration that may recover with abstinence. Reactive, increased adult neurogenesis during abstinence following alcohol dependence may contribute to recovery, but the mechanism driving reactive neurogenesis is not known. Therefore, adult, male rats were exposed to alcohol for four days and various markers were used to examine cell cycle dynamics, the percentage and number of neural progenitor cell subtypes, and the percentage of quiescent versus activated progenitors. Using a screen for cell cycle perturbation, we showed that the cell cycle is not likely altered at 7 days in abstinence. As the vast majority of Bromodeoxyuridine-positive (+) cells were co-labeled with progenitor cell marker, Sox2, we then developed a quadruple fluorescent labeling scheme to examine Type-1, -2a, -2b and -3 progenitor cells simultaneously. Prior alcohol dependence indiscriminately increased all subtypes at 7 days, the peak of the reactive proliferation. An evaluation of the time course of reactive cell proliferation revealed that cells begin proliferating at 5 days post alcohol, where only actively dividing Type 2 progenitors were increased by alcohol. Furthermore, prior alcohol increased the percentage of actively dividing Sox2+ progenitors, which supported that reactive neurogenesis is likely due to the activation of progenitors out of quiescence. These observations were associated with granule cell number returning to normal at 28 days. Therefore, activating stem and progenitor cells out of quiescence may be the mechanism underlying hippocampal recovery in abstinence following alcohol dependence.
Keywords: Adult neurogenesis; Alcoholism; Ethanol; Hippocampus; Neurodegeneration; Progenitor cell.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
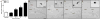


Similar articles
-
Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model.Hippocampus. 2010 May;20(5):596-607. doi: 10.1002/hipo.20665. Hippocampus. 2010. PMID: 19554644 Free PMC article.
-
Type 2 Neural Progenitor Cell Activation Drives Reactive Neurogenesis after Binge-Like Alcohol Exposure in Adolescent Male Rats.Front Psychiatry. 2017 Dec 15;8:283. doi: 10.3389/fpsyt.2017.00283. eCollection 2017. Front Psychiatry. 2017. PMID: 29326611 Free PMC article.
-
Ectopic hippocampal neurogenesis in adolescent male rats following alcohol dependence.Addict Biol. 2014 Jul;19(4):687-99. doi: 10.1111/adb.12075. Epub 2013 Jul 11. Addict Biol. 2014. PMID: 23844726 Free PMC article.
-
Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism.Hippocampus. 2006;16(3):287-95. doi: 10.1002/hipo.20162. Hippocampus. 2006. PMID: 16421863 Review.
-
Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Oct 3;54:103-13. doi: 10.1016/j.pnpbp.2014.05.003. Epub 2014 May 17. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24842804 Free PMC article. Review.
Cited by
-
Functional Activation of Newborn Neurons Following Alcohol-Induced Reactive Neurogenesis.Brain Sci. 2021 Apr 15;11(4):499. doi: 10.3390/brainsci11040499. Brain Sci. 2021. PMID: 33921189 Free PMC article.
-
Consequences of adolescent alcohol use on adult hippocampal neurogenesis and hippocampal integrity.Int Rev Neurobiol. 2021;160:281-304. doi: 10.1016/bs.irn.2021.08.005. Epub 2021 Oct 6. Int Rev Neurobiol. 2021. PMID: 34696876 Free PMC article. Review.
-
Reactive, Adult Neurogenesis From Increased Neural Progenitor Cell Proliferation Following Alcohol Dependence in Female Rats.Front Neurosci. 2021 Sep 14;15:689601. doi: 10.3389/fnins.2021.689601. eCollection 2021. Front Neurosci. 2021. PMID: 34594180 Free PMC article.
-
Effects of Chronic Voluntary Alcohol Drinking on Thiamine Concentrations, Endoplasmic Reticulum Stress, and Oxidative Stress in the Brain of Crossed High Alcohol Preferring Mice.Neurotox Res. 2019 Nov;36(4):777-787. doi: 10.1007/s12640-019-00032-y. Epub 2019 Apr 10. Neurotox Res. 2019. PMID: 30972556 Free PMC article.
-
The effect of chronic alcohol exposure on spatial memory and BDNF-TrkB- PLCγ1 signaling in the hippocampus of male and female mice.Heliyon. 2023 May 30;9(6):e16660. doi: 10.1016/j.heliyon.2023.e16660. eCollection 2023 Jun. Heliyon. 2023. PMID: 37303582 Free PMC article.
References
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. Journal of Comparative Neurology. 1969;136:269–293. - PubMed
-
- Bartels C, Kunert HJ, Stawicki S, Kroner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol Alcohol. 2007;42:92–102. - PubMed
-
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, Liu D, Shen D, Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–1870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

