Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception
- PMID: 29378387
- PMCID: PMC5933892
- DOI: 10.4062/biomolther.2017.127
Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception
Abstract
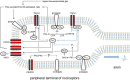
Inflammation is one of the main causes of pathologic pain. Knowledge of the molecular links between inflammatory signals and pain-mediating neuronal signals is essential for understanding the mechanisms behind pain exacerbation. Some inflammatory mediators directly modulate the excitability of pain-mediating neurons by contacting the receptor molecules expressed in those neurons. For decades, many discoveries have accumulated regarding intraneuronal signals from receptor activation through electrical depolarization for bradykinin, a major inflammatory mediator that is able to both excite and sensitize pain-mediating nociceptor neurons. Here, we focus on the final effectors of depolarization, the neuronal ion channels, whose functionalities are specifically affected by bradykinin stimulation. Particular G-protein coupled signaling cascades specialized for each specific depolarizer ion channels are summarized. Some of these ion channels not only serve as downstream effectors but also play critical roles in relaying specific pain modalities such as thermal or mechanical pain. Accordingly, specific pain phenotypes altered by bradykinin stimulation are also discussed. Some members of the effector ion channels are both activated and sensitized by bradykinin-induced neuronal signaling, while others only sensitized or inhibited, which are also introduced. The present overview of the effect of bradykinin on nociceptor neuronal excitability at the molecular level may contribute to better understanding of an important aspect of inflammatory pain and help future design of further research on the components involved and pain modulating strategies.
Keywords: Bradykinin; Depolarization; Ion channel; Nociceptor neuron; Pain.
Figures
References
-
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

