Early animal model evaluation of an implantable contrast agent to enhance magnetic resonance imaging of arterial bypass vein grafts
- PMID: 29378421
- PMCID: PMC7439609
- DOI: 10.1177/0284185117753656
Early animal model evaluation of an implantable contrast agent to enhance magnetic resonance imaging of arterial bypass vein grafts
Abstract
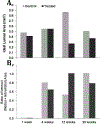
Background Non-invasive monitoring of autologous vein graft (VG) bypass grafts is largely limited to detecting late luminal narrowing. Although magnetic resonance imaging (MRI) delineates vein graft intima, media, and adventitia, which may detect early failure, the scan time required to achieve sufficient resolution is at present impractical. Purpose To study VG visualization enhancement in vivo and delineate whether a covalently attached MRI contrast agent would enable quicker longitudinal imaging of the VG wall. Material and Methods Sixteen 12-week-old male C57BL/6J mice underwent carotid interposition vein grafting. The inferior vena cava of nine donor mice was treated with a gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA)-based contrast agent, with control VGs labeled with a vehicle. T1-weighted (T1W) MRI was performed serially at postoperative weeks 1, 4, 12, and 20. A portion of animals was sacrificed for histopathology following each imaging time point. Results MRI signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were significantly higher for treated VGs in the first three time points (1.73 × higher SNR, P = 0.0006, and 5.83 × higher CNR at the first time point, P = 0.0006). However, MRI signal enhancement decreased consistently in the study period, to 1.29 × higher SNR and 2.64 × higher CNR, by the final time point. There were no apparent differences in graft morphometric analyses in Masson's trichrome-stained sections. Conclusion A MRI contrast agent that binds covalently to the VG wall provides significant increase in T1W MRI signal with no observed adverse effects in a mouse model. Further optimization of the contrast agent to enhance its durability is required.
Keywords: Magnetic resonance imaging; contrast media; gadolinium; image enhancement; peripheral vascular diseases; signal-to-noise ratio; vascular grafting.
Conflict of interest statement
Figures





Similar articles
-
Mouse model of venous bypass graft arteriosclerosis.Am J Pathol. 1998 Oct;153(4):1301-10. doi: 10.1016/S0002-9440(10)65675-1. Am J Pathol. 1998. PMID: 9777962 Free PMC article.
-
Different signal intensity at Gd-EOB-DTPA compared with Gd-DTPA-enhanced MRI in hepatocellular carcinoma transgenic mouse model in delayed phase hepatobiliary imaging.J Magn Reson Imaging. 2012 Jun;35(6):1397-402. doi: 10.1002/jmri.23584. Epub 2012 Jan 20. J Magn Reson Imaging. 2012. PMID: 22267126
-
Comparison of ferumoxytol- and gadolinium chelate-enhanced MRI for assessment of sarcomas in children and adolescents.Eur Radiol. 2020 Mar;30(3):1790-1803. doi: 10.1007/s00330-019-06569-y. Epub 2019 Dec 16. Eur Radiol. 2020. PMID: 31844962 Free PMC article.
-
Neointimal hyperplasia rapidly reaches steady state in a novel murine vein graft model.J Vasc Surg. 2002 Oct;36(4):824-32. J Vasc Surg. 2002. PMID: 12368745
-
Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA.Invest Radiol. 2009 Jun;44(6):305-10. doi: 10.1097/rli.0b013e3181a24512. Invest Radiol. 2009. PMID: 19462484
References
-
- Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. JVascSurg. 2006; 43: 742–51. - PubMed
-
- Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005; 294: 2446–54. - PubMed
-
- Berceli SA, Hevelone ND, Lipsitz SR, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. Journal of vascular surgery. 2007; 46: 1173–9. - PubMed
-
- Kirby PL, Brady AR, Thompson SG, Torgerson D and Davies AH. The Vein Graft Surveillance Trial: rationale, design and methods. VGST participants. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 1999; 18: 469–74. - PubMed
-
- Baldus S, Koster R, Elsner M, et al. Treatment of aortocoronary vein graft lesions with membrane-covered stents: A multicenter surveillance trial. Circulation. 2000; 102: 2024–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

