Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-κB and MAPK pathways
- PMID: 29378573
- PMCID: PMC5789647
- DOI: 10.1186/s12917-018-1360-0
Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-κB and MAPK pathways
Abstract
Background: The uteruses of most dairy cattle are easily infected by bacteria, especially gram-negative bacteria, following parturition. Macrophages are important cells of the immune system and play a critical role in the inflammatory response. In addition, cortisol levels become significantly increased due to the stress of parturition in dairy cattle, and cortisol is among the most widely used and effective therapies for many inflammatory diseases. In this study, we assessed the anti-inflammatory effects and potential molecular mechanisms of cortisol using a Lipopolysaccharide (LPS)-induced RAW264.7 macrophage cell line.
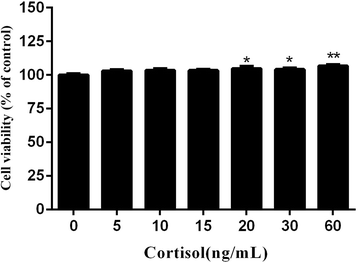
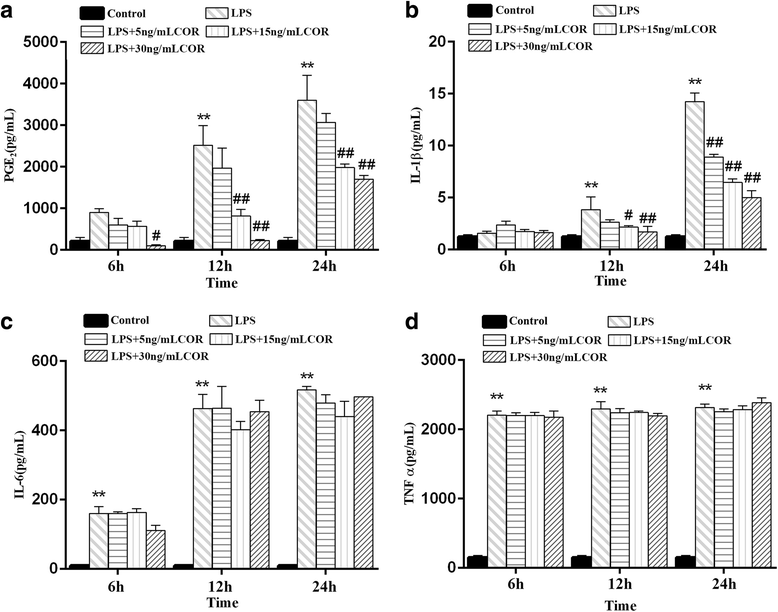
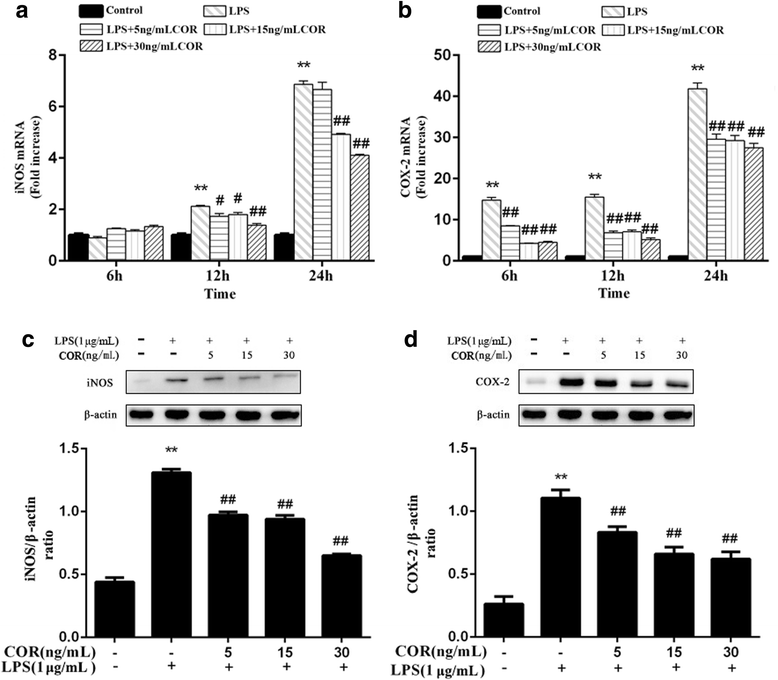
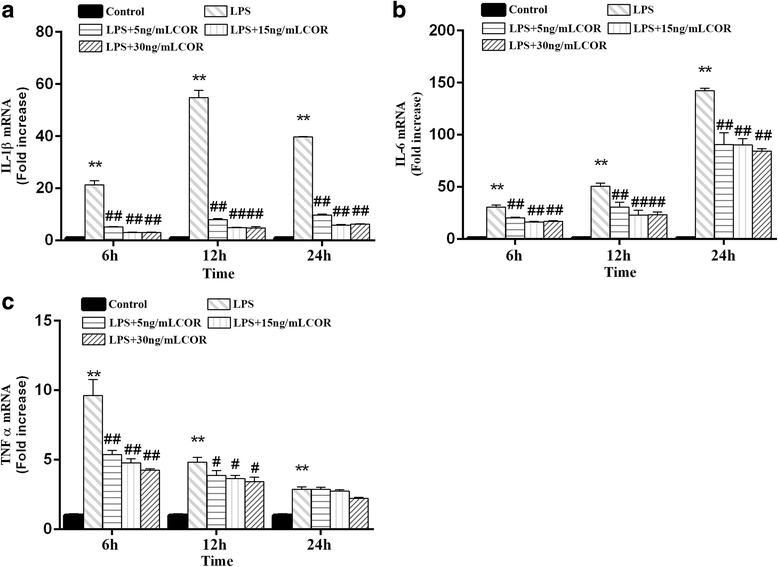
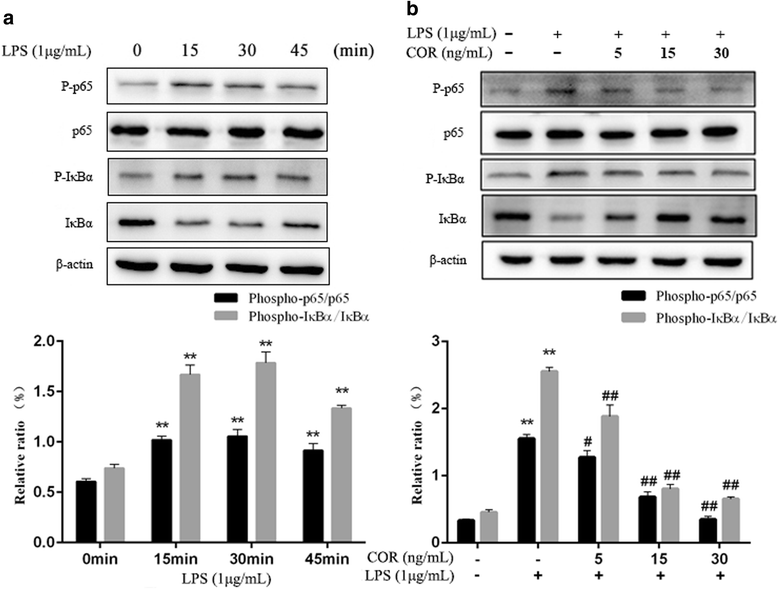
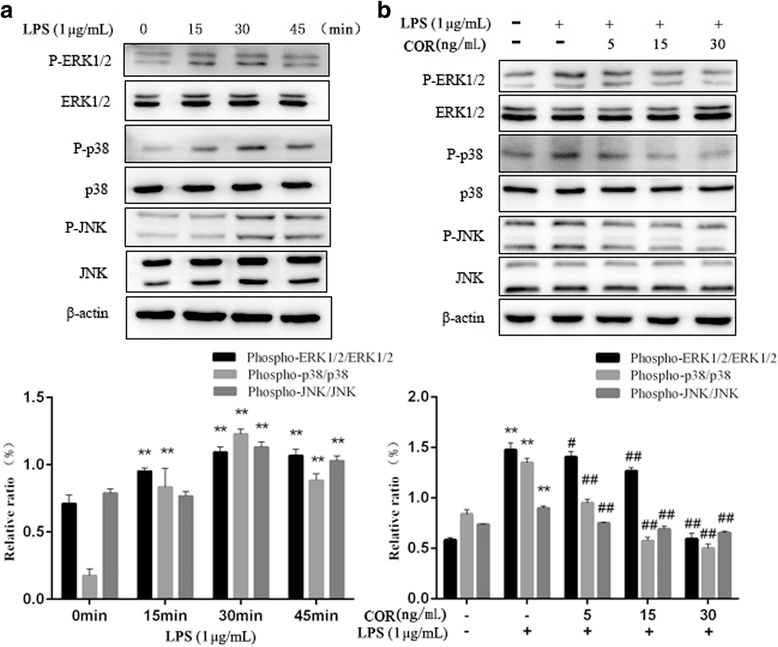
Results: Cortisol significantly suppressed the production of prostaglandin E2 (PGE2) and decreased the gene and protein expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) in a dose-dependent manner. Moreover, cortisol inhibited the mRNA expression of pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β), and interleukin-6 (IL-6) and decreased IL-1β secretion in an LPS-treated RAW264.7 macrophage cell line. Moreover, we found that cortisol suppressed nuclear factor-kappa B (NF-κB) signaling in RAW264.7 macrophages stimulated with LPS. This suppression was mediated by the inhibition of IκBα degradation and NF-κB p65 phosphorylation. In addition, cortisol also suppressed the phosphorylation of mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinase (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK).
Conclusions: These results suggest that high cortisol levels can attenuate LPS-induced inflammatory responses in the RAW264.7 macrophage cell line by regulating the NF-κB and MAPK signaling pathways.
Keywords: Anti-inflammatory; Cortisol; LPS; MAPKs; Macrophage; NF-κB.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kim KS, Lee DS, Bae GS, Park SJ, Kang DG, Lee HS, Oh H, Kim YC. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside a isolated from Polygala Tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 2013;721(1–3):267–276. doi: 10.1016/j.ejphar.2013.09.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- No.31672614,31072176, 31302151/the National Science Foundation of China
- No.BK20160062, BK2012265/Natural Science Foundation of Jiangsu Province
- 17KJB230007/Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China
- No.31502127/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

