Plasmodium vivax molecular diagnostics in community surveys: pitfalls and solutions
- PMID: 29378609
- PMCID: PMC5789620
- DOI: 10.1186/s12936-018-2201-0
Plasmodium vivax molecular diagnostics in community surveys: pitfalls and solutions
Abstract
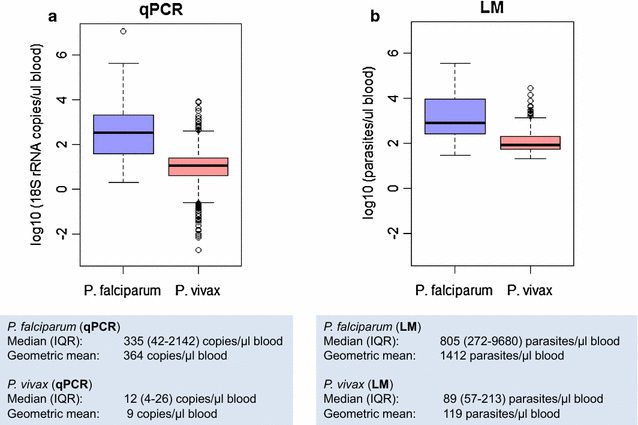
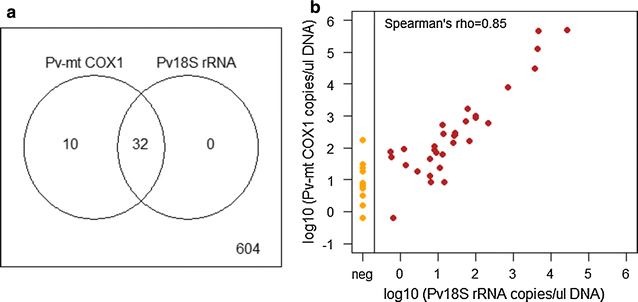
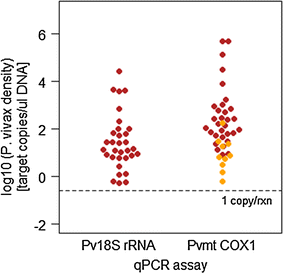
A distinctive feature of Plasmodium vivax infections is the overall low parasite density in peripheral blood. Thus, identifying asymptomatic infected individuals in endemic communities requires diagnostic tests with high sensitivity. The detection limits of molecular diagnostic tests are primarily defined by the volume of blood analysed and by the copy number of the amplified molecular marker serving as the template for amplification. By using mitochondrial DNA as the multi-copy template, the detection limit can be improved more than tenfold, compared to standard 18S rRNA targets, thereby allowing detection of lower parasite densities. In a very low transmission area in Brazil, application of a mitochondrial DNA-based assay increased prevalence from 4.9 to 6.5%. The usefulness of molecular tests in malaria epidemiological studies is widely recognized, especially when precise prevalence rates are desired. Of concern, however, is the challenge of demonstrating test accuracy and quality control for samples with very low parasite densities. In this case, chance effects in template distribution around the detection limit constrain reproducibility. Rigorous assessment of false positive and false negative test results is, therefore, required to prevent over- or under-estimation of parasite prevalence in epidemiological studies or when monitoring interventions.
Keywords: 18S rRNA transcripts; Gametocytes; LAMP; Mitochondrial DNA; Molecular diagnostics; Plasmodium vivax; Quantification; Surveillance.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

