Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres
- PMID: 29378675
- PMCID: PMC5818250
- DOI: 10.7554/eLife.31299
Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres
Abstract
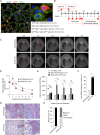
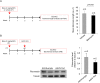
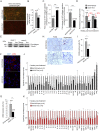
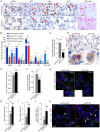
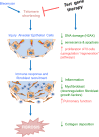
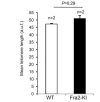
Pulmonary fibrosis is a fatal lung disease characterized by fibrotic foci and inflammatory infiltrates. Short telomeres can impair tissue regeneration and are found both in hereditary and sporadic cases. We show here that telomerase expression using AAV9 vectors shows therapeutic effects in a mouse model of pulmonary fibrosis owing to a low-dose bleomycin insult and short telomeres. AAV9 preferentially targets regenerative alveolar type II cells (ATII). AAV9-Tert-treated mice show improved lung function and lower inflammation and fibrosis at 1-3 weeks after viral treatment, and improvement or disappearance of the fibrosis at 8 weeks after treatment. AAV9-Tert treatment leads to longer telomeres and increased proliferation of ATII cells, as well as lower DNA damage, apoptosis, and senescence. Transcriptome analysis of ATII cells confirms downregulation of fibrosis and inflammation pathways. We provide a proof-of-principle that telomerase activation may represent an effective treatment for pulmonary fibrosis provoked or associated with short telomeres.
Keywords: AAV9; chromosomes; gene therapy; genes; mouse; pulmonary fibrosis; telomerase; telomeres.
© 2018, Povedano et al.
Conflict of interest statement
JP, PM, RS, ÁT, GG, JF, FB, MB No competing interests declared, MB is an employee for F. Hoffmann-La Roche Ltd, and the author declares no other competing financial interests
Figures

References
-
- Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, Tuder RM, Armanios M. Telomere length is a determinant of emphysema susceptibility. American Journal of Respiratory and Critical Care Medicine. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

