The Axon Initial Segment: An Updated Viewpoint
- PMID: 29378864
- PMCID: PMC6596274
- DOI: 10.1523/JNEUROSCI.1922-17.2018
The Axon Initial Segment: An Updated Viewpoint
Abstract
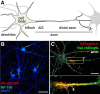
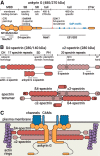
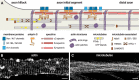
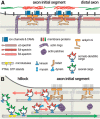
At the base of axons sits a unique compartment called the axon initial segment (AIS). The AIS generates and shapes the action potential before it is propagated along the axon. Neuronal excitability thus depends crucially on the AIS composition and position, and these adapt to developmental and physiological conditions. The AIS also demarcates the boundary between the somatodendritic and axonal compartments. Recent studies have brought insights into the molecular architecture of the AIS and how it regulates protein trafficking. This Viewpoints article summarizes current knowledge about the AIS and highlights future challenges in understanding this key actor of neuronal physiology.
Copyright © 2018 the authors 0270-6474/18/382135-11$15.00/0.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
