PhoPR Positively Regulates whiB3 Expression in Response to Low pH in Pathogenic Mycobacteria
- PMID: 29378889
- PMCID: PMC5869482
- DOI: 10.1128/JB.00766-17
PhoPR Positively Regulates whiB3 Expression in Response to Low pH in Pathogenic Mycobacteria
Abstract
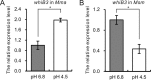
During infection, Mycobacterium tuberculosis colonizes macrophages or necrotic granulomas, in which low pH is one of the major challenges. The PhoPR two-component regulatory system and the cytosolic redox sensor WhiB3 both play important roles in the response to low pH by M. tuberculosis However, whether close association exists between PhoPR and WhiB3 remains unclear. In this study, the positive regulation of whiB3 by PhoPR in mycobacteria was characterized. We observed that the expression patterns of the whiB3 gene under acidic conditions are different among mycobacterial species, suggesting that the regulation of whiB3 differs among mycobacteria. A sequence analysis of the whiB3 promoters (whiB3p) from M. tuberculosis and two closely related species, namely, M. marinum and M. smegmatis, showed that the whiB3p regions from M. tuberculosis and M. marinum contain a new type of PhoP box that is absent in the M. smegmatiswhiB3p Direct binding of PhoP to whiB3p from M. tuberculosis and M. marinum but not that from M. smegmatis was validated by in vitro protein-DNA binding assays. The direct activation of whiB3 by PhoPR under acidic conditions was further verified by reverse transcription-quantitative PCR (qRT-PCR) analysis in M. marinum Moreover, mutating the residues important for the phosphorylation pathway of PhoPR in M. marinum abolished the activation of whiB3 expression by PhoPR under acidic conditions, suggesting that low pH triggers the phosphorylation of PhoPR, which in turn activates the transcription of whiB3 Since the PhoP box was only identified in whiB3p of pathogenic mycobacteria, we suggest that the PhoPR-whiB3 regulatory pathway may have evolved to facilitate mycobacterial infection.IMPORTANCE The low pH in macrophages is an important barrier for infection by microbes. The PhoPR two-component regulatory system is required for the response to low pH and plays a role in redox homeostasis in Mycobacterium tuberculosis WhiB3, a cytosolic redox-sensing transcriptional regulator, is also involved in these processes. However, there is no direct evidence to demonstrate the regulation of WhiB3 by PhoPR. In this study, we found that PhoPR directly activates whiB3 expression in response to low pH. An atypical PhoP box in the whiB3 promoters has been identified and is only found in pathogenic mycobacteria, which suggests that the PhoPR-whiB3 regulatory pathway may facilitate mycobacterial infection. This study provides novel information for further characterization of the PhoPR regulon.
Keywords: Mycobacterium; transcriptional regulation; two-component regulatory systems.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
An ArsR-like transcriptional factor recognizes a conserved sequence motif and positively regulates the expression of phoP in mycobacteria.Biochem Biophys Res Commun. 2011 Aug 12;411(4):726-31. doi: 10.1016/j.bbrc.2011.07.014. Epub 2011 Jul 18. Biochem Biophys Res Commun. 2011. PMID: 21782791
-
EspM Is a Conserved Transcription Factor That Regulates Gene Expression in Response to the ESX-1 System.mBio. 2020 Feb 4;11(1):e02807-19. doi: 10.1128/mBio.02807-19. mBio. 2020. PMID: 32019792 Free PMC article.
-
Characterization of the two component regulatory system PhoPR in Mycobacterium bovis.Vet Microbiol. 2018 Aug;222:30-38. doi: 10.1016/j.vetmic.2018.06.016. Epub 2018 Jun 21. Vet Microbiol. 2018. PMID: 30080670
-
Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development.mBio. 2015 Oct 20;6(5):e01289-15. doi: 10.1128/mBio.01289-15. mBio. 2015. PMID: 26489860 Free PMC article. Review.
-
[Mycobacterium tuberculosis PhoP system].Wei Sheng Wu Xue Bao. 2017 Apr 4;57(4):461-7. Wei Sheng Wu Xue Bao. 2017. PMID: 29756729 Review. Chinese.
Cited by
-
Two-component sensor histidine kinases of Mycobacterium tuberculosis: Beacons for niche navigation.Mol Microbiol. 2022 May;117(5):973-985. doi: 10.1111/mmi.14899. Epub 2022 Apr 11. Mol Microbiol. 2022. PMID: 35338720 Free PMC article. Review.
-
Unraveling Mycobacterium tuberculosis acid resistance and pH homeostasis mechanisms.FEBS Lett. 2025 Jun;599(12):1634-1648. doi: 10.1002/1873-3468.70023. Epub 2025 Feb 24. FEBS Lett. 2025. PMID: 39995204 Free PMC article. Review.
-
Acid Fasting: Modulation of Mycobacterium tuberculosis Metabolism at Acidic pH.Trends Microbiol. 2019 Nov;27(11):942-953. doi: 10.1016/j.tim.2019.06.005. Epub 2019 Jul 16. Trends Microbiol. 2019. PMID: 31324436 Free PMC article. Review.
-
Gene Regulatory Mechanism of Mycobacterium Tuberculosis during Dormancy.Curr Issues Mol Biol. 2024 Jun 11;46(6):5825-5844. doi: 10.3390/cimb46060348. Curr Issues Mol Biol. 2024. PMID: 38921019 Free PMC article. Review.
-
Targeting Mycobacterium tuberculosis pH-driven adaptation.Microbiology (Reading). 2024 May;170(5):001458. doi: 10.1099/mic.0.001458. Microbiology (Reading). 2024. PMID: 38717801 Free PMC article. Review.
References
-
- Gouzy A, Larrouy-Maumus G, Bottai D, Levillain F, Dumas A, Wallach JB, Caire-Brandli I, de Chastellier C, Wu TD, Poincloux R, Brosch R, Guerquin-Kern JL, Schnappinger D, Sorio de Carvalho LP, Poquet Y, Neyrolles O. 2014. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog 10:e1003928. doi:10.1371/journal.ppat.1003928. - DOI - PMC - PubMed
-
- Small JL, O'Donoghue AJ, Boritsch EC, Tsodikov OV, Knudsen GM, Vandal O, Craik CS, Ehrt S. 2013. Substrate specificity of MarP, a periplasmic protease required for resistance to acid and oxidative stress in Mycobacterium tuberculosis. J Biol Chem 288:12489–12499. doi:10.1074/jbc.M113.456541. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

