E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells
- PMID: 29378943
- PMCID: PMC5816191
- DOI: 10.1073/pnas.1718185115
E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells
Abstract
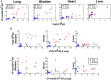
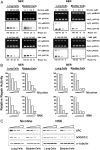
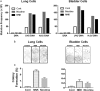
E-cigarette smoke delivers stimulant nicotine as aerosol without tobacco or the burning process. It contains neither carcinogenic incomplete combustion byproducts nor tobacco nitrosamines, the nicotine nitrosation products. E-cigarettes are promoted as safe and have gained significant popularity. In this study, instead of detecting nitrosamines, we directly measured DNA damage induced by nitrosamines in different organs of E-cigarette smoke-exposed mice. We found mutagenic O6-methyldeoxyguanosines and γ-hydroxy-1,N2 -propano-deoxyguanosines in the lung, bladder, and heart. DNA-repair activity and repair proteins XPC and OGG1/2 are significantly reduced in the lung. We found that nicotine and its metabolite, nicotine-derived nitrosamine ketone, can induce the same effects and enhance mutational susceptibility and tumorigenic transformation of cultured human bronchial epithelial and urothelial cells. These results indicate that nicotine nitrosation occurs in vivo in mice and that E-cigarette smoke is carcinogenic to the murine lung and bladder and harmful to the murine heart. It is therefore possible that E-cigarette smoke may contribute to lung and bladder cancer, as well as heart disease, in humans.
Keywords: DNA damage; DNA repair; E-cigarettes; cancer; lung–bladder–heart.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Tobacco smoking, E-cigarettes, and nicotine harm.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):1406-1407. doi: 10.1073/pnas.1722636115. Epub 2018 Jan 31. Proc Natl Acad Sci U S A. 2018. PMID: 29386385 Free PMC article. No abstract available.
-
Reply to Li Volti et al.: E-cigarette smoke exposure and effect in mice and human cells.Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):E3075-E3076. doi: 10.1073/pnas.1802912115. Epub 2018 Mar 13. Proc Natl Acad Sci U S A. 2018. PMID: 29535225 Free PMC article. No abstract available.
-
Assessment of E-cigarette impact on smokers: The importance of experimental conditions relevant to human consumption.Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):E3073-E3074. doi: 10.1073/pnas.1801967115. Epub 2018 Mar 13. Proc Natl Acad Sci U S A. 2018. PMID: 29535226 Free PMC article. No abstract available.
-
Reply to Queimado et al.: E-cigarettes induce DNA damage and inhibit DNA repair in mice and human cells.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5439. doi: 10.1073/pnas.1807971115. Epub 2018 May 25. Proc Natl Acad Sci U S A. 2018. PMID: 29802232 Free PMC article. No abstract available.
-
Electronic cigarette aerosols induce DNA damage and reduce DNA repair: Consistency across species.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5437-E5438. doi: 10.1073/pnas.1807411115. Epub 2018 May 25. Proc Natl Acad Sci U S A. 2018. PMID: 29802233 Free PMC article. No abstract available.
-
Re: E-Cigarette Smoke Damages DNA and Reduces Repair Activity in Mouse Lung, Heart, and Bladder as Well as in Human Lung and Bladder Cells.J Urol. 2018 Oct;200(4):701. doi: 10.1016/j.juro.2018.07.003. Epub 2018 Jul 14. J Urol. 2018. PMID: 30227585 No abstract available.
References
-
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

