Preferential amplification of a human mitochondrial DNA deletion in vitro and in vivo
- PMID: 29379065
- PMCID: PMC5789095
- DOI: 10.1038/s41598-018-20064-2
Preferential amplification of a human mitochondrial DNA deletion in vitro and in vivo
Abstract
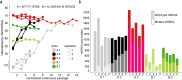
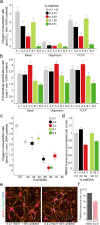
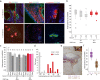
We generated induced pluripotent stem cells (iPSCs) from patient fibroblasts to yield cell lines containing varying degrees of heteroplasmy for a m.13514 A > G mtDNA point mutation (2 lines) and for a ~6 kb single, large scale mtDNA deletion (3 lines). Long term culture of the iPSCs containing a single, large-scale mtDNA deletion showed consistent increase in mtDNA deletion levels with time. Higher levels of mtDNA heteroplasmy correlated with increased respiratory deficiency. To determine what changes occurred in deletion level during differentiation, teratomas comprising all three embryonic germ layers were generated from low (20%) and intermediate heteroplasmy (55%) mtDNA deletion clones. Regardless of whether iPSCs harbouring low or intermediate mtDNA heteroplasmy were used, the final levels of heteroplasmy in all teratoma germ layers increased to a similar high level (>60%). Thus, during human stem cell division, cells not only tolerate high mtDNA deletion loads but seem to preferentially replicate deleted mtDNA genomes. This has implications for the involvement of mtDNA deletions in both disease and ageing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- G0800674/MRC_/Medical Research Council/United Kingdom
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- BB/E012841/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0301182/MRC_/Medical Research Council/United Kingdom
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

