A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy
- PMID: 29379121
- PMCID: PMC5788981
- DOI: 10.1038/s41598-018-20233-3
A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy
Abstract
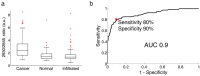
Modern cancer diagnosis requires histological, molecular, and genomic tumor analyses. Tumor sampling is often achieved using a targeted needle biopsy approach. Targeting errors and cancer heterogeneity causing inaccurate sampling are important limitations of this blind technique leading to non-diagnostic or poor quality samples, and the need for repeated biopsies pose elevated patient risk. An optical technology that can analyze the molecular nature of the tissue prior to harvesting could improve cancer targeting and mitigate patient risk. Here we report on the design, development, and validation of an in situ intraoperative, label-free, cancer detection system based on high wavenumber Raman spectroscopy. This optical detection device was engineered into a commercially available biopsy system allowing tumor analysis prior to tissue harvesting without disrupting workflow. Using a dual validation approach we show that high wavenumber Raman spectroscopy can detect human dense cancer with >60% cancer cells in situ during surgery with a sensitivity and specificity of 80% and 90%, respectively. We also demonstrate for the first time the use of this system in a swine brain biopsy model. These studies set the stage for the clinical translation of this optical molecular imaging method for high yield and safe targeted biopsy.
Conflict of interest statement
K.P., F.L., E.M, and K.U. are co-founders of ODS Medical Inc, a medical device company that seeks to commercialize the Raman spectroscopy system for real time detection of tissue abnormalities.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

