Local control of intracellular microtubule dynamics by EB1 photodissociation
- PMID: 29379139
- PMCID: PMC5826794
- DOI: 10.1038/s41556-017-0028-5
Local control of intracellular microtubule dynamics by EB1 photodissociation
Abstract
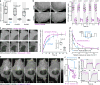
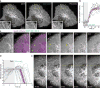
End-binding proteins (EBs) are adaptors that recruit functionally diverse microtubule plus-end-tracking proteins (+TIPs) to growing microtubule plus ends. To test with high spatial and temporal accuracy how, when and where +TIP complexes contribute to dynamic cell biology, we developed a photo-inactivated EB1 variant (π-EB1) by inserting a blue-light-sensitive protein-protein interaction module between the microtubule-binding and +TIP-binding domains of EB1. π-EB1 replaces endogenous EB1 function in the absence of blue light. By contrast, blue-light-mediated π-EB1 photodissociation results in rapid +TIP complex disassembly, and acutely and reversibly attenuates microtubule growth independent of microtubule end association of the microtubule polymerase CKAP5 (also known as ch-TOG and XMAP215). Local π-EB1 photodissociation allows subcellular control of microtubule dynamics at the second and micrometre scale, and elicits aversive turning of migrating cancer cells. Importantly, light-mediated domain splitting can serve as a template to optically control other intracellular protein activities.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

