Exploration of the role of the virulence factor ElrA during Enterococcus faecalis cell infection
- PMID: 29379180
- PMCID: PMC5788860
- DOI: 10.1038/s41598-018-20206-6
Exploration of the role of the virulence factor ElrA during Enterococcus faecalis cell infection
Abstract
Enterococcus faecalis, an organism generally not pathogenic for healthy humans, has the potential to cause disease in susceptible hosts. While it seems to be equipped to interact with and circumvent host immune defense, most of the molecular and cellular mechanisms underlying the enterococcal infectious process remain elusive. Here, we investigated the role of the Enterococcal Leucine Rich protein A (ElrA), an internalin-like protein of E. faecalis also known as a virulence factor. ElrA was previously shown to prevent adhesion to macrophages. We show that ElrA does not inhibit the basic phagocytic process, but is able to prevent sensing and migration of macrophages toward E. faecalis. Presence or absence of FHL2, a eukaryotic partner of ElrA, does not affect the ElrA-dependent mechanism preventing macrophage migration. However, we highlight a partial contribution of FHL2 in ElrA-mediated virulence in vivo. Our results indicate that ElrA plays at least a dual role of which anti-phagocytic activity may contribute to dissemination of extracellular E. faecalis during infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
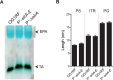

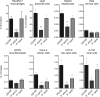
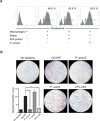
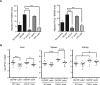
References
-
- Sievert DM, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

