Adherence to riluzole in patients with amyotrophic lateral sclerosis: an observational study
- PMID: 29379292
- PMCID: PMC5757977
- DOI: 10.2147/NDT.S150550
Adherence to riluzole in patients with amyotrophic lateral sclerosis: an observational study
Abstract
Objective: Riluzole is the first drug approved to treat amyotrophic lateral sclerosis (ALS). Recently, an oral suspension (OS) of riluzole was made available. Thus, the aim of our study was to evaluate the adherence to 2 formulations of riluzole in patients with ALS.
Patients and methods: We enrolled 45 consecutive patients with ALS. At disease diagnosis, riluzole was prescribed in 2 different formulations depending on the severity of dysphagia (27/45 patients received tablets and 18/45 patients received OS). Side effects (SEs) and treatment adherence were investigated using a clinical questionnaire including the ©Morisky 8-item Medication Adherence Questionnaire.
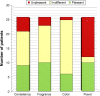
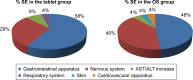
Results: Gastroenteric complaints were the most frequent SEs (58% in the tablet group and 48% in the OS group), followed by those at the nervous system (29% and 40%, respectively). No serious SEs related to treatment were reported. The rate of adherence to riluzole was independent of the formulation of the drug and consistent with other medications assumed for comorbidities (p=0.004). In the tablet group, low adherence was caused by SEs in 55.6% and by dysphagia in 44.4% of patients. In the OS group, SEs caused low adherence in 75% of patients. Independently of the drug formulation, patients with high or medium adherence to riluzole had a higher progression rate (p=0.002 and p=0.009, respectively) and a shorter time to generalization (TTG; p=0.01), compared to those with low adherence.
Conclusion: Gastroenteric symptoms were the most frequent SE related to tablet as well as OS. The rate of adherence was independent of the formulation of riluzole and the number of medications assumed for comorbidities, and it was consistent with the severity of the disease. The low adherence was caused by dysphagia and SEs in the tablet group, whereas it was caused prevalently by SEs in the OS group.
Keywords: adherence; oral suspension; riluzole; side effects; tablet.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390(10107):2084–2098. - PubMed
-
- Bensimon G, Lacomblez L, Meininger V, ALS/Riluzole Study Group A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330(9):585–591. - PubMed
-
- Lacomblez L, Bensimon G, Leigh PN, et al. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology. 1996;47(6 Suppl 4):S242–S250. - PubMed
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V, for the Amyotrophic Lateral Sclerosis/Riluzole Study Group II Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347(9013):1425–1431. - PubMed
-
- Zoccolella S, Beghi E, Palagano G, et al. SLAP Registry Riluzole and amyotrophic lateral sclerosis survival: a population-based study in southern Italy. Eur J Neurol. 2007;14(3):262–268. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

