Dendrobium officinale Orchid Extract Prevents Ovariectomy-Induced Osteoporosis in Vivo and Inhibits RANKL-Induced Osteoclast Differentiation in Vitro
- PMID: 29379436
- PMCID: PMC5775521
- DOI: 10.3389/fphar.2017.00966
Dendrobium officinale Orchid Extract Prevents Ovariectomy-Induced Osteoporosis in Vivo and Inhibits RANKL-Induced Osteoclast Differentiation in Vitro
Erratum in
-
Corrigendum: Dendrobium officinale Orchid Extract Prevents Ovariectomy-Induced Osteoporosis In Vivo and Inhibits RANKL-Induced Osteoclast Differentiation In Vitro.Front Pharmacol. 2020 Apr 30;11:578. doi: 10.3389/fphar.2020.00578. eCollection 2020. Front Pharmacol. 2020. PMID: 32425797 Free PMC article.
Abstract
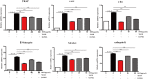
Background:Dendrobium officinale, a traditional Chinese medical herb with high value that is widely used in Asia, possesses many positive effects on human health, including anti-chronic inflammation, anti-obesity, and immune modulation properties; however, whether D. officinale has inhibitory effects on postmenopausal osteoporosis remains unknown. Objective: We investigated the effects of D. officinale extract (DOE) on ovariectomy-induced bone loss in vivo and on osteoclastogenesis in vitro. Methods:In vivo, female rats were divided into a sham-operated (sham) group and five ovariectomized (OVX) subgroups: OVX with vehicle (OVX), OVX with Xian-Ling-Gu-Bao capsule (240 mg/kg body weight/day), and OVX with low-, medium-, and high-dose DOE (150, 300, and 600 mg/kg body weight/day, respectively). Animals in each group were administered their corresponding treatments for 13 weeks. Body weight, serum biochemical parameters, uterine and femoral physical parameters, bone mineral density (BMD), bone biomechanical properties, and bone microarchitecture were obtained. In vitro, the effects of DOE on osteoclastogenesis were examined using RAW264.7 cells. The effects of DOE on osteoclastogenesis and the expression of osteoclast-specific marker genes and proteins were determined. Results: DOE effectively ameliorated serum biochemical parameters, especially alleviated estradiol (E2) deficiency and maintained calcium and phosphorus homeostasis. DOE improved uterine and femoral physical parameters. In addition, DOE improved femoral BMD and biomechanical properties. DOE significantly ameliorated bone microarchitecture. Moreover, DOE inhibited osteoclastogenesis independent of its cytoxicity and suppressed the expression of osteoclast-specific marker genes and proteins. Conclusion: DOE can effectively prevent ovariectomy-induced bone loss in vivo and inhibit osteoclastogenesis in vitro.
Keywords: DOE; bone quality; osteoclastogenesis; ovariectomy; postmenopausal osteoporosis.
Figures






References
-
- Baek J. M., Kim J. Y., Ahn S. J., Cheon Y. H., Yang M., Oh J., et al. (2016). Dendrobium moniliforme exerts inhibitory effects on both receptor activator of nuclear factor Kappa-B ligand-mediated osteoclast differentiation in vitro and lipopolysaccharide-induced bone erosion in vivo. Molecules 21:295. 10.3390/molecules21030295 - DOI - PMC - PubMed
-
- Chen X. M., Xiao S. Y., Guo S. X. (2006). Comparison of chemical compositions between Dendrobium candidum and Dendrobium nobile. Acta Acad. Med. Sin. 28 524–529. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

