Progress in Mathematical Modeling of Gastrointestinal Slow Wave Abnormalities
- PMID: 29379448
- PMCID: PMC5775268
- DOI: 10.3389/fphys.2017.01136
Progress in Mathematical Modeling of Gastrointestinal Slow Wave Abnormalities
Abstract
Gastrointestinal (GI) motility is regulated in part by electrophysiological events called slow waves, which are generated by the interstitial cells of Cajal (ICC). Slow waves propagate by a process of "entrainment," which occurs over a decreasing gradient of intrinsic frequencies in the antegrade direction across much of the GI tract. Abnormal initiation and conduction of slow waves have been demonstrated in, and linked to, a number of GI motility disorders. A range of mathematical models have been developed to study abnormal slow waves and applied to propose novel methods for non-invasive detection and therapy. This review provides a general outline of GI slow wave abnormalities and their recent classification using multi-electrode (high-resolution) mapping methods, with a particular emphasis on the spatial patterns of these abnormal activities. The recently-developed mathematical models are introduced in order of their biophysical scale from cellular to whole-organ levels. The modeling techniques, main findings from the simulations, and potential future directions arising from notable studies are discussed.
Keywords: Electrophysiology; GI; arrhythmias; multi-scale modeling; slow wave.
Figures
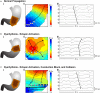
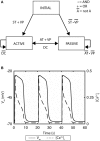
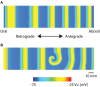


References
-
- Angeli T. R., Cheng L. K., Du P., Wang T. H., Bernard C. E., Vannucchi M. G., et al. . (2015). Loss of interstitial cells of cajal and patterns of gastric dysrhythmia in patients with chronic unexplained nausea and vomiting. Gastroenterology 149, 56-66 e5. 10.1053/j.gastro.2015.04.003 - DOI - PMC - PubMed
-
- Angeli T. R., O'Grady G., Paskaranandavadivel N., Erickson J. C., Du P., Pullan A. J., et al. . (2013b). Experimental and automated analysis techniques for high resolution electrical mapping of small intestine slow wave activity. J. Neurogastroenterol. Motil. 19, 179–191. 10.5056/jnm.2013.19.2.179 - DOI - PMC - PubMed
-
- Atia J., McCloskey C., Shmygol A. S., Rand D. A. H. A., van den Berg Blanks A. M. (2016). Reconstruction of cell surface densities of ion pumps, exchangers, and channels from mRNA expression, conductance kinetics, whole-cell calcium, and current-clamp voltage recordings, with an application to human uterine smooth muscle cells. PLoS Comput. Biol. 12:e1004828. 10.1371/journal.pcbi.1004828 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

