Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy
- PMID: 29379493
- PMCID: PMC5770808
- DOI: 10.3389/fimmu.2017.01751
Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy
Abstract
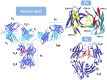
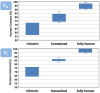
The remarkable progress in engineering and clinical development of therapeutic antibodies in the last 40 years, after the seminal work by Köhler and Milstein, has led to the approval by the United States Food and Drug Administration (FDA) of 21 antibodies for cancer immunotherapy. We review here these approved antibodies, with emphasis on the methods used for their discovery, engineering, and optimization for therapeutic settings. These methods include antibody engineering via chimerization and humanization of non-human antibodies, as well as selection and further optimization of fully human antibodies isolated from human antibody phage-displayed libraries and immunization of transgenic mice capable of generating human antibodies. These technology platforms have progressively led to the development of therapeutic antibodies with higher human content and, thus, less immunogenicity. We also discuss the genetic engineering approaches that have allowed isotype switching and Fc modifications to modulate effector functions and bioavailability (half-life), which together with the technologies for engineering the Fv fragment, have been pivotal in generating more efficacious and better tolerated therapeutic antibodies to treat cancer.
Keywords: Fc engineering; chimerization; humanization; oncology; phage display; therapeutic antibodies; transgenic mice.
Figures


References
-
- Emmons C, Hunsicker LG. Muromonab-CD3 (Orthoclone OKT3): the first monoclonal antibody approved for therapeutic use. Iowa Med (1987) 77(2):78–82. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

