Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS
- PMID: 29379497
- PMCID: PMC5760512
- DOI: 10.3389/fimmu.2017.01835
Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS
Abstract
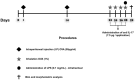
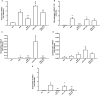
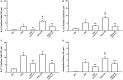
Inflammation plays a central role in the development of asthma, which is considered an allergic disease with a classic Th2 inflammatory profile. However, cytokine IL-17 has been examined to better understand the pathophysiology of this disease. Severe asthmatic patients experience frequent exacerbations, leading to infection, and subsequently show altered levels of inflammation that are unlikely to be due to the Th2 immune response alone. This study estimates the effects of anti-IL-17 therapy in the pulmonary parenchyma in a murine asthma model exacerbated by LPS. BALB/c mice were sensitized with intraperitoneal ovalbumin and repeatedly exposed to inhalation with ovalbumin, followed by treatment with or without anti-IL-17. Twenty-four hours prior to the end of the 29-day experimental protocol, the two groups received LPS (0.1 mg/ml intratracheal OVA-LPS and OVA-LPS IL-17). We subsequently evaluated bronchoalveolar lavage fluid, performed a lung tissue morphometric analysis, and measured IL-6 gene expression. OVA-LPS-treated animals treated with anti-IL-17 showed decreased pulmonary inflammation, edema, oxidative stress, and extracellular matrix remodeling compared to the non-treated OVA and OVA-LPS groups (p < 0.05). The anti-IL-17 treatment also decreased the numbers of dendritic cells, FOXP3, NF-κB, and Rho kinase 1- and 2-positive cells compared to the non-treated OVA and OVA-LPS groups (p < 0.05). In conclusion, these data suggest that inhibition of IL-17 is a promising therapeutic avenue, even in exacerbated asthmatic patients, and significantly contributes to the control of Th1/Th2/Th17 inflammation, chemokine expression, extracellular matrix remodeling, and oxidative stress in a murine experimental asthma model exacerbated by LPS.
Keywords: LPS-exacerbated; anti-IL-17; asthma; distal lung; inflammation.
Figures







References
-
- GINA. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) (2015). Available from: http://www.ginaasthma.org
LinkOut - more resources
Full Text Sources
Other Literature Sources

