Photocatalytic formation of carbon-sulfur bonds
- PMID: 29379578
- PMCID: PMC5769090
- DOI: 10.3762/bjoc.14.4
Photocatalytic formation of carbon-sulfur bonds
Abstract
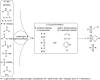
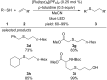
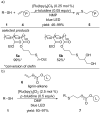
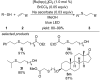
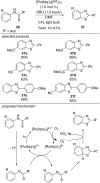
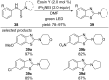
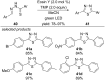
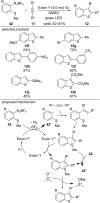
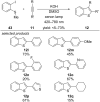
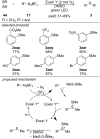
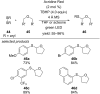
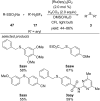
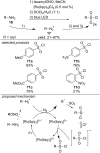
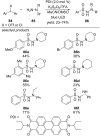
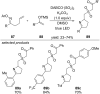
This review summarizes recent developments in photocatalyzed carbon-sulfur bond formation. General concepts, synthetic strategies and the substrate scope of reactions yielding thiols, disulfides, sulfoxides, sulfones and other organosulfur compounds are discussed together with the proposed mechanistic pathways.
Keywords: disulfides; photocatalysis; sulfones; sulfoxides; thiols; visible light.
Figures

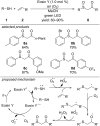
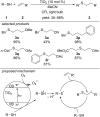
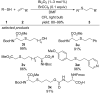


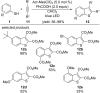
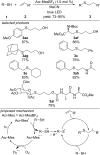
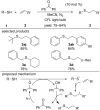



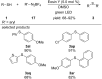
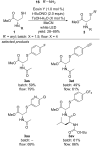

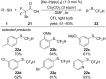
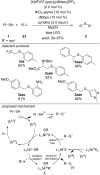
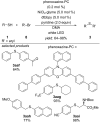
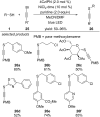
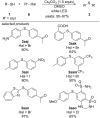

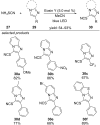

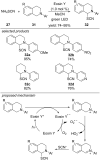
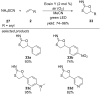





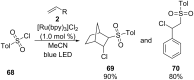
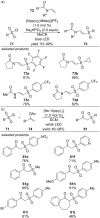
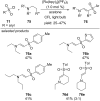
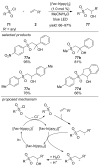
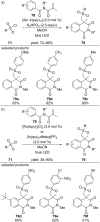

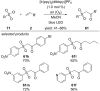
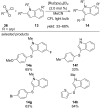

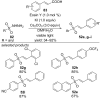
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
