Pioglitazone and cause-specific risk of mortality in patients with type 2 diabetes: extended analysis from a European multidatabase cohort study
- PMID: 29379607
- PMCID: PMC5783110
- DOI: 10.1136/bmjdrc-2017-000481
Pioglitazone and cause-specific risk of mortality in patients with type 2 diabetes: extended analysis from a European multidatabase cohort study
Abstract
Objectives: Describe and compare the risk of cardiovascular and non-cardiovascular mortality in patients whose antidiabetic therapy is modified to include pioglitazone compared with an alternative antidiabetic medication at the same stage of disease progression.
Research design and methods: This exploratory linked database cohort analysis used pooled health and mortality data from three European countries: Finland, Sweden and the UK. Propensity score together with exact matching was used to match 31 133 patients with type 2 diabetes first prescribed pioglitazone from 2000 to 2011, to 31 133 patients never prescribed pioglitazone. Exact matching variables were treatment stage, history of diabetes, diabetes complications and cardiovascular disease, and year of cohort entry. Mean follow-up time was 2.60 (SD 2.00) and 2.69 (SD 2.31) years in the pioglitazone and non-pioglitazone-exposed groups, respectively. Crude cause-specific mortality rates were ascertained. Association with pioglitazone use was estimated using Cox proportional hazards models adjusted a priori for country, age, sex, the propensity score quintile and time-dependent variables representing use of antidiabetic drugs. Stepwise testing identified no additional confounders to include in adjusted models.
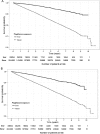
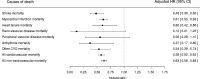
Results: The crude mortality rate was lower in the pioglitazone-exposed group than the non-exposed group for both cardiovascular and non-cardiovascular mortality. Adjusted HRs comparing pioglitazone to alternative antidiabetic exposure were 0.58 (95% CI 0.52 to 0.63) and 0.63 (95% CI 0.58 to 0.68) for cardiovascular and non-cardiovascular mortality, respectively. A protective effect associated with pioglitazone was also found for all specific cardiovascular causes.
Conclusions: This analysis suggests that pioglitazone is associated with a decrease in both cardiovascular and non-cardiovascular mortality. Results should be interpreted with caution due to the potential for residual confounding in this exploratory analysis. Further studies, specifically designed to test the association between pioglitazone use and patient-focused outcomes, are suggested.
Study registration number: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; EUPAS3626).
Keywords: Cardiovacsular Disease(s); Pioglitazone; mortality.
Conflict of interest statement
Competing interests: PK, FH, SC and MM are employed by EPID Research, EMH is employed by PHARMO Institute, RW and HS are employed by CPRD, and ML and SB are employed by Karolinska Institute. EPID Research, PHARMO Institute, CPRD and the Centre for Pharmacoepidemiology (CPE) at Karolinska Institute perform commissioned pharmacoepidemiological studies and thus their employees have been and currently are working in collaboration with several pharmaceutical companies (including Takeda). DB is employed by Takeda Pharmaceutical Company Limited.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
