Efficacy of Oral Mixed Tocotrienols in Diabetic Peripheral Neuropathy: A Randomized Clinical Trial
- PMID: 29379943
- PMCID: PMC5885181
- DOI: 10.1001/jamaneurol.2017.4609
Efficacy of Oral Mixed Tocotrienols in Diabetic Peripheral Neuropathy: A Randomized Clinical Trial
Abstract
Importance: Management of painful diabetic peripheral neuropathy remains challenging. Most therapies provide symptomatic relief with varying degrees of efficacy. Tocotrienols have modulatory effects on the neuropathy pathway and may reduce neuropathic symptoms with their antioxidative and anti-inflammatory activities.
Objective: To evaluate the efficacy of oral mixed tocotrienols for patients with diabetic peripheral neuropathy.
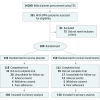
Design, setting, and participants: The Vitamin E in Neuroprotection Study (VENUS) was a parallel, double-blind, placebo-controlled trial that recruited participants from January 30, 2011, to December 7, 2014, with 12 months of follow-up. This trial screened 14 289 patients with diabetes from 6 health clinics and ambulatory care units from 5 public hospitals in Malaysia. A total of 391 patients who reported neuropathic symptoms were further assessed with Total Symptom Score (TSS) and Neuropathy Impairment Score (NIS). Patients 20 years or older with a TSS of 3 or higher and an NIS of 2 or higher were recruited.
Interventions: Patients were randomized to receive 200 mg of mixed tocotrienols twice daily or matching placebo for 12 months. Patients with hyperhomocysteinemia (homocysteine level ≥2.03 mg/L) received oral folic acid, 5 mg once daily, and methylcobalamin, 500 μg thrice daily, in both groups.
Main outcomes and measures: The primary outcome was patient-reported neuropathy TSS (lancinating pain, burning pain, paresthesia, and asleep numbness) changes at 12 months. The secondary outcomes were NIS and sensory nerve conduction test result.
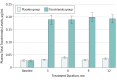
Results: Of 391 eligible patients, 300 were recruited (130 [43.3%] male; mean [SD] age, 57.6 [8.9] years; mean [SD] duration of diabetes, 11.4 [7.8] years) and 229 (76.3%) completed the trial. The TSS changes between the tocotrienols and placebo groups at 12 months (-0.30; 95% CI, -1.16 to 0.56; P = .49) were similar. No significant differences in NIS (0.60; 95% CI, -1.37 to 2.65; P = .53) and sensory nerve conduction test assessments were found between both groups. In post hoc subgroup analyses, tocotrienols reduced lancinating pain among patients with hemoglobin A1C levels greater than 8% (P = .03) and normohomocysteinemia (homocysteine level <2.03 mg/L; P = .008) at 1 year. Serious adverse events in both groups were similar, except more infections were observed in the tocotrienols group (6.7% vs 0.7%, P = .04). Results reported were of modified intention-to-treat analyses.
Conclusions and relevance: Supplementation of oral mixed tocotrienols, 400 mg/d for 1 year, did not improve overall neuropathic symptoms. The preliminary observations on lancinating pain among subsets of patients require further exploration.
Trial registration: National Medical Research Registry Identifier: NMRR-10-948-7327 and clinicaltrials.gov Identifier: NCT01973400.
Conflict of interest statement
Figures
References
-
- Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480-1481. - PubMed
-
- Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(suppl 2):S50-S62. - PubMed
-
- Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29(2):212-217. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical