Effect of an In-Hospital Multifaceted Clinical Pharmacist Intervention on the Risk of Readmission: A Randomized Clinical Trial
- PMID: 29379953
- PMCID: PMC5885912
- DOI: 10.1001/jamainternmed.2017.8274
Effect of an In-Hospital Multifaceted Clinical Pharmacist Intervention on the Risk of Readmission: A Randomized Clinical Trial
Abstract
Importance: Hospital readmissions are common among patients receiving multiple medications, with considerable costs to the patients and society.
Objective: To determine whether a multifaceted pharmacist intervention based on medication review, patient interview, and follow-up can reduce the number of readmissions and emergency department (ED) visits.
Design, setting, and participants: This randomized clinical multicenter study (Odense Pharmacist Trial Investigating Medication Interventions at Sector Transfer [OPTIMIST]) enrolled patients from September 1, 2013, through April 23, 2015, with a follow-up of 6 months completed on October 31, 2015. Consecutive medical patients in an acute admission ward who were 18 years or older and who used 5 or more medications were invited to participate. Of 1873 patients invited to participate, 1499 (80.0%) accepted. The medication review and patient interview were conducted in the hospital and followed up in collaboration with primary care. Analysis was based on intention to treat.
Interventions: The patients were randomized into 3 groups receiving usual care (no intervention), a basic intervention (medication review), and an extended intervention (medication review, 3 motivational interviews, and follow-up with the primary care physician, pharmacy, and nursing home).
Main outcomes and measures: The prespecified primary outcomes were readmission within 30 or 180 days and ED visits within 180 days. The primary composite end point was readmission or an ED visit within 180 days. Secondary outcomes were drug-related readmissions within 30 and 180 days after inclusion, and all-cause mortality and drug-related mortality.
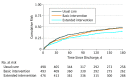
Results: A total of 1467 patients (679 men [46.3%] and 788 women [53.7%]; median age, 72 years; interquartile range, 63-80 years) were part of the primary analysis, including 498 randomized to usual care, 493 randomized to the basic intervention, and 476 randomized to the extended intervention. The extended intervention had a significant effect on the numbers of patients who were readmitted within 30 days (hazard ratio [HR], 0.62; 95% CI, 0.46-0.84) or within 180 days (HR, 0.75; 95% CI, 0.62-0.90) after inclusion and on the number of patients who experienced the primary composite end point (HR, 0.77; 95% CI, 0.64-0.93). The study showed a nonsignificant reduction in drug-related readmissions within 30 days (HR, 0.65; 95% CI, 0.39-1.09) and within 180 days (HR, 0.80; 95% CI, 0.59-1.08) after inclusion and in deaths (HR, 0.83; 95% CI, 0.22-3.11). The number needed to treat to achieve the primary composite outcome for the extended intervention (vs usual care) was 12.
Conclusions and relevance: A multifaceted clinical pharmacist intervention may reduce the number of ED visits and hospital readmissions.
Trial registration: clinicaltrials.gov Identifier: NCT03079375.
Conflict of interest statement
Figures
Comment in
-
In hospitalized adults with polypharmacy, a multifaceted pharmacist intervention reduced readmissions at 180 days.Ann Intern Med. 2018 May 15;168(10):JC59. doi: 10.7326/ACPJC-2018-168-10-059. Ann Intern Med. 2018. PMID: 29800435 No abstract available.
-
Enough Power to Build a Strong Case for Clinical Pharmacy Services?JAMA Intern Med. 2018 Jun 1;178(6):864. doi: 10.1001/jamainternmed.2018.1706. JAMA Intern Med. 2018. PMID: 29868735 No abstract available.
-
Enough Power to Build a Strong Case for Clinical Pharmacy Services?-Reply.JAMA Intern Med. 2018 Jun 1;178(6):864-865. doi: 10.1001/jamainternmed.2018.1709. JAMA Intern Med. 2018. PMID: 29868748 No abstract available.
References
-
- Beijer HJM, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46-54. - PubMed
-
- Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017-1025. - PubMed
-
- Cunningham G, Dodd TR, Grant DJ, McMurdo ME, Richards RM. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing. 1997;26(5):375-382. - PubMed
-
- Mannesse CK, Derkx FH, de Ridder MA, Man in ’t Veld AJ, van der Cammen TJ. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29(1):35-39. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

