Cross talk between β subunits, intracellular Ca2+ signaling, and SNAREs in the modulation of CaV 2.1 channel steady-state inactivation
- PMID: 29380539
- PMCID: PMC5789719
- DOI: 10.14814/phy2.13557
Cross talk between β subunits, intracellular Ca2+ signaling, and SNAREs in the modulation of CaV 2.1 channel steady-state inactivation
Abstract
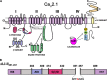
Modulation of CaV 2.1 channel activity plays a key role in interneuronal communication and synaptic plasticity. SNAREs interact with a specific synprint site at the second intracellular loop (LII-III) of the CaV 2.1 pore-forming α1A subunit to optimize neurotransmitter release from presynaptic terminals by allowing secretory vesicles docking near the Ca2+ entry pathway, and by modulating the voltage dependence of channel steady-state inactivation. Ca2+ influx through CaV 2.1 also promotes channel inactivation. This process seems to involve Ca2+ -calmodulin interaction with two adjacent sites in the α1A carboxyl tail (C-tail) (the IQ-like motif and the Calmodulin-Binding Domain (CBD) site), and contributes to long-term potentiation and spatial learning and memory. Besides, binding of regulatory β subunits to the α interaction domain (AID) at the first intracellular loop (LI-II) of α1A determines the degree of channel inactivation by both voltage and Ca2+ . Here, we explore the cross talk between β subunits, Ca2+ , and syntaxin-1A-modulated CaV 2.1 inactivation, highlighting the α1A domains involved in such process. β3 -containing CaV 2.1 channels show syntaxin-1A-modulated but no Ca2+ -dependent steady-state inactivation. Conversely, β2a -containing CaV 2.1 channels show Ca2+ -dependent but not syntaxin-1A-modulated steady-state inactivation. A LI-II deletion confers Ca2+ -dependent inactivation and prevents modulation by syntaxin-1A in β3 -containing CaV 2.1 channels. Mutation of the IQ-like motif, unlike CBD deletion, abolishes Ca2+ -dependent inactivation and confers modulation by syntaxin-1A in β2a -containing CaV 2.1 channels. Altogether, these results suggest that LI-II structural modifications determine the regulation of CaV 2.1 steady-state inactivation either by Ca2+ or by SNAREs but not by both.
Keywords: Ca2+-calmodulin; CaV2.1 domains for SNARE-mediated modulation; CaV2.1 steady-state inactivation; CaVβ subunits; presynaptic voltage-gated CaV2.1 channels; syntaxin-1A.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures








References
-
- Atlas, D. 2013. The voltage‐gated calcium channel functions as the molecular switch of synaptic transmission. Annu. Rev. Biochem. 82:607–635. - PubMed
-
- Bezprozvanny, I. , Scheller R. H., and Tsien R. W.. 1995. Functional impact of syntaxin on gating of N‐type and Q‐type calcium channels. Nature 378:623–626. - PubMed
-
- Birnbaumer, L. , Qin N., Olcese R., Tareilus E., Platano D., Costantin J., et al. 1998. Structures and functions of calcium channel β subunits. J. Bioenerg. Biomembr. 30:357–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

