A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II
- PMID: 29381090
- PMCID: PMC5867508
- DOI: 10.1089/dia.2017.0142
A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II
Abstract
Background: Persistent use of real-time continuous glucose monitoring (CGM) improves diabetes control in individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D).
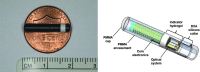
Methods: PRECISE II was a nonrandomized, blinded, prospective, single-arm, multicenter study that evaluated the accuracy and safety of the implantable Eversense CGM system among adult participants with T1D and T2D (NCT02647905). The primary endpoint was the mean absolute relative difference (MARD) between paired Eversense and Yellow Springs Instrument (YSI) reference measurements through 90 days postinsertion for reference glucose values from 40 to 400 mg/dL. Additional endpoints included Clarke Error Grid analysis and sensor longevity. The primary safety endpoint was the incidence of device-related or sensor insertion/removal procedure-related serious adverse events (SAEs) through 90 days postinsertion.
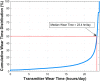
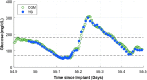
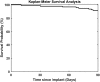
Results: Ninety participants received the CGM system. The overall MARD value against reference glucose values was 8.8% (95% confidence interval: 8.1%-9.3%), which was significantly lower than the prespecified 20% performance goal for accuracy (P < 0.0001). Ninety-three percent of CGM values were within 20/20% of reference values over the total glucose range of 40-400 mg/dL. Clarke Error Grid analysis showed 99.3% of samples in the clinically acceptable error zones A (92.8%) and B (6.5%). Ninety-one percent of sensors were functional through day 90. One related SAE (1.1%) occurred during the study for removal of a sensor.
Conclusions: The PRECISE II trial demonstrated that the Eversense CGM system provided accurate glucose readings through the intended 90-day sensor life with a favorable safety profile.
Keywords: Accuracy; Continuous glucose monitoring; Implantable; Longevity; Type 1 diabetes; Type 2 diabetes.
Conflict of interest statement
M.P.C., A.R.C., D.L., D.S.D., and G.A. report no disclosures. L.J.K. has received research grants from Medtronic, Abbott, and Dexcom. R.B. has received research funding from Senseonics, Medtronic, Dexcom, Abbott, Roche Diabetes Care, Sanofi, Lilly, Novo, Viromed, Diasome, and Novartis. C.J.L. has received research support from Senseonics and Lexicon Pharmaceuticals, both paid directly to the institution. B.W.B. has received speaker and consultant fees from Astra Zeneca, BI/Lilly, Janssen, Medtronic, Novo Nordisk, and Sanofi; his employer (Atlanta Diabetes Associates) has received research and grant support from Abbott, Dexcom, Medtronic, and Senseonics. T.S.B. reports the following disclosures: research support from Abbott, Ambra, Ascensia, BD, Boehringer Ingelheim, Calibra, Companion Medical, Dexcom, Elcelyx, Glysens, Janssen, Lexicon, Lilly, Medtronic, Novo Nordisk, Sanofi, Senseonics, Versartis, and Xeris; consulting honoraria from Astra Zeneca, Bayer, BD, Calibra, Lilly, Medtronic, Novo Nordisk, and Sanofi; and speaking honoraria from Abbott, Insulet, Medtronic, Lilly, Novo Nordisk, and Sanofi.
Figures


Similar articles
-
A Prospective Multicenter Evaluation of the Accuracy and Safety of an Implanted Continuous Glucose Sensor: The PRECISION Study.Diabetes Technol Ther. 2019 May;21(5):231-237. doi: 10.1089/dia.2019.0020. Epub 2019 Mar 29. Diabetes Technol Ther. 2019. PMID: 30925083 Free PMC article.
-
Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial.Diabetes Care. 2017 Jan;40(1):63-68. doi: 10.2337/dc16-1525. Epub 2016 Nov 4. Diabetes Care. 2017. PMID: 27815290 Clinical Trial.
-
Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study.Diabetes Technol Ther. 2022 Feb;24(2):84-92. doi: 10.1089/dia.2021.0182. Epub 2021 Sep 9. Diabetes Technol Ther. 2022. PMID: 34515521 Free PMC article.
-
A Review of the First Long-term Implantable Continuous Glucose Monitoring System Available in the United States.J Diabetes Sci Technol. 2021 Jan;15(1):160-166. doi: 10.1177/1932296819890865. Epub 2019 Dec 13. J Diabetes Sci Technol. 2021. PMID: 31833388 Free PMC article. Review.
-
CONTINUOUS GLUCOSE MONITORING: A CONSENSUS CONFERENCE OF THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY.Endocr Pract. 2016 Aug;22(8):1008-21. doi: 10.4158/EP161392.CS. Epub 2016 May 23. Endocr Pract. 2016. PMID: 27214060
Cited by
-
From Point-of-Care Testing to eHealth Diagnostic Devices (eDiagnostics).ACS Cent Sci. 2018 Dec 26;4(12):1600-1616. doi: 10.1021/acscentsci.8b00625. Epub 2018 Nov 20. ACS Cent Sci. 2018. PMID: 30648144 Free PMC article. Review.
-
Longitudinal Analysis of Real-World Performance of an Implantable Continuous Glucose Sensor over Multiple Sensor Insertion and Removal Cycles.Diabetes Technol Ther. 2020 May;22(5):422-427. doi: 10.1089/dia.2019.0342. Diabetes Technol Ther. 2020. PMID: 31697182 Free PMC article.
-
Current treatment options and challenges in patients with Type 1 diabetes: Pharmacological, technical advances and future perspectives.Rev Endocr Metab Disord. 2021 Jun;22(2):217-240. doi: 10.1007/s11154-021-09635-3. Epub 2021 Mar 23. Rev Endocr Metab Disord. 2021. PMID: 33755854 Free PMC article. Review.
-
Cutaneous Complications With Continuous or Flash Glucose Monitoring Use: Systematic Review of Trials and Observational Studies.J Diabetes Sci Technol. 2020 Mar;14(2):328-337. doi: 10.1177/1932296819870849. Epub 2019 Aug 27. J Diabetes Sci Technol. 2020. PMID: 31452386 Free PMC article.
-
12th Roche Diabetes Care Network Meeting: April 11-13, 2019, Copenhagen, Denmark.Diabetes Technol Ther. 2020 Feb;22(2):142-167. doi: 10.1089/dia.2019.0392. Epub 2020 Jan 14. Diabetes Technol Ther. 2020. PMID: 31692374 Free PMC article.
References
-
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 - PMC - PubMed
-
- O'Connell MA, Donath S, O'Neal DN, et al. : Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
