Time-to-event modelling of effect of codrituzumab on overall survival in patients with hepatocellular carcinoma
- PMID: 29381229
- PMCID: PMC5903238
- DOI: 10.1111/bcp.13530
Time-to-event modelling of effect of codrituzumab on overall survival in patients with hepatocellular carcinoma
Abstract
Aims: Codrituzumab (GC33) is a recombinant, humanized mAb that binds to glypican-3 (GPC3), an oncofetal protein highly expressed in hepatocellular carcinoma (HCC). This investigation aimed to identify clinically relevant factors that may affect the overall survival (OS) in HCC patients treated with codrituzumab and to quantitatively annotate their effects.
Methods: Codrituzumab exposure was estimated by a population pharmacokinetics model with a nonlinear elimination pathway. Analysis of OS was performed using a time-to-event model in 181 patients with advanced HCC. The model was tested with the addition of various covariates, including levels of immune biomarkers, such as CD16 (measured in terms of molecules of equivalent soluble fluorophore; CD16MESF ) and CD4, codrituzumab exposure and potential prognostic biomarkers of HCC such as baseline tumour size and soluble GPC3.
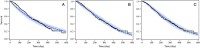
Results: The time-to-event model estimated a prolonged OS (>3 months) in patients with codrituzumab exposure of ≥230 μg ml-1 and high CD16MESF level (>5.26 × 105 MESF at least). The Weibull model was selected as the base hazard model. The baseline tumour size was included in the hazard model as a parameter independent of the drug effect. A logistic model was applied to explain the effects of drug exposure and CD16MESF level.
Conclusions: The final model indicates that adequate drug exposure plus a favourable immune environment are associated with prolonged OS. This quantitative model should be further validated with emerging data so as to guide study design in future clinical trials.
Keywords: GC33/codrituzumab; GPC3; hepatocellular carcinoma; overall survival; pharmacokinetics; time-to-event model.
© 2018 The British Pharmacological Society.
Figures

Similar articles
-
Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma.J Hepatol. 2016 Aug;65(2):289-95. doi: 10.1016/j.jhep.2016.04.004. Epub 2016 Apr 13. J Hepatol. 2016. PMID: 27085251 Clinical Trial.
-
Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC).Cancer Chemother Pharmacol. 2017 Feb;79(2):421-429. doi: 10.1007/s00280-017-3241-9. Epub 2017 Jan 24. Cancer Chemother Pharmacol. 2017. PMID: 28120036 Free PMC article. Clinical Trial.
-
Case-control Indian buffet process identifies biomarkers of response to Codrituzumab.BMC Cancer. 2019 Mar 28;19(1):278. doi: 10.1186/s12885-019-5472-0. BMC Cancer. 2019. PMID: 30922327 Free PMC article. Clinical Trial.
-
Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma.World J Gastroenterol. 2016 Jan 7;22(1):275-83. doi: 10.3748/wjg.v22.i1.275. World J Gastroenterol. 2016. PMID: 26755876 Free PMC article. Review.
-
Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment.Med Res Rev. 2018 Mar;38(2):741-767. doi: 10.1002/med.21455. Epub 2017 Jun 16. Med Res Rev. 2018. PMID: 28621802 Review.
Cited by
-
Immunotherapeutic Targeting of GPC3 in Pediatric Solid Embryonal Tumors.Front Oncol. 2019 Feb 26;9:108. doi: 10.3389/fonc.2019.00108. eCollection 2019. Front Oncol. 2019. PMID: 30873384 Free PMC article. Review.
References
-
- Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, et al Anti‐glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2009; 378: 279–284. - PubMed
-
- Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, et al Anti‐glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res 2008; 68: 9832–9838. - PubMed
-
- Zhu AX, Gold PJ, El‐Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, et al First‐in‐man phase I study of GC33, a novel recombinant humanized antibody against glypican‐3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2013; 19: 920–928. - PubMed
-
- Abou‐Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, et al Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol 2016; 65: 289–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

