Selective Inhibition of Nuclear Export With Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma
- PMID: 29381435
- PMCID: PMC6905485
- DOI: 10.1200/JCO.2017.75.5207
Selective Inhibition of Nuclear Export With Oral Selinexor for Treatment of Relapsed or Refractory Multiple Myeloma
Abstract
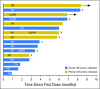
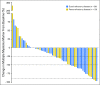
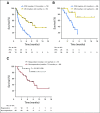
Purpose Selinexor, a first-in-class, oral, selective exportin 1 (XPO1) inhibitor, induces apoptosis in cancer cells through nuclear retention of tumor suppressor proteins and the glucocorticoid receptor, along with inhibition of translation of oncoprotein mRNAs. We studied selinexor in combination with low-dose dexamethasone in patients with multiple myeloma refractory to the most active available agents. Patients and Methods This phase II trial evaluated selinexor 80 mg and dexamethasone 20 mg, both orally and twice weekly, in patients with myeloma refractory to bortezomib, carfilzomib, lenalidomide, and pomalidomide (quad-refractory disease), with a subset also refractory to an anti-CD38 antibody (penta-refractory disease). The primary end point was overall response rate (ORR). Results Of 79 patients, 48 had quad-refractory and 31 had penta-refractory myeloma. Patients had received a median of seven prior regimens. The ORR was 21% and was similar for patients with quad-refractory (21%) and penta-refractory (20%) disease. Among patients with high-risk cytogenetics, including t(4;14), t(14;16), and del(17p), the ORR was 35% (six of 17 patients). The median duration of response was 5 months, and 65% of responding patients were alive at 12 months. The most common grade ≥ 3 adverse events were thrombocytopenia (59%), anemia (28%), neutropenia (23%), hyponatremia (22%), leukopenia (15%), and fatigue (15%). Dose interruptions for adverse events occurred in 41 patients (52%), dose reductions occurred in 29 patients (37%), and treatment discontinuation occurred in 14 patients (18%). Conclusion The combination of selinexor and dexamethasone has an ORR of 21% in patients with heavily pretreated, refractory myeloma with limited therapeutic options.
Trial registration: ClinicalTrials.gov NCT02336815.
Figures
Similar articles
-
Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma.N Engl J Med. 2019 Aug 22;381(8):727-738. doi: 10.1056/NEJMoa1903455. N Engl J Med. 2019. PMID: 31433920 Clinical Trial.
-
Selinexor: A First-in-Class Nuclear Export Inhibitor for Management of Multiply Relapsed Multiple Myeloma.Ann Pharmacother. 2020 Jun;54(6):577-582. doi: 10.1177/1060028019892643. Epub 2019 Dec 2. Ann Pharmacother. 2020. PMID: 31793336 Free PMC article. Review.
-
Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma.Blood. 2018 Dec 13;132(24):2546-2554. doi: 10.1182/blood-2018-06-858852. Epub 2018 Oct 23. Blood. 2018. PMID: 30352784 Free PMC article. Clinical Trial.
-
Selinexor for the treatment of multiple myeloma.Expert Opin Pharmacother. 2020 Mar;21(4):399-408. doi: 10.1080/14656566.2019.1707184. Epub 2020 Jan 19. Expert Opin Pharmacother. 2020. PMID: 31957504 Review.
-
Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma.Br J Haematol. 2019 Aug;186(4):549-560. doi: 10.1111/bjh.15969. Epub 2019 May 24. Br J Haematol. 2019. PMID: 31124580 Free PMC article. Clinical Trial.
Cited by
-
Drug resistance in multiple myeloma: Soldiers and weapons in the bone marrow niche.Front Oncol. 2022 Sep 21;12:973836. doi: 10.3389/fonc.2022.973836. eCollection 2022. Front Oncol. 2022. PMID: 36212502 Free PMC article. Review.
-
Safety and activity of selinexor in patients with myelodysplastic syndromes or oligoblastic acute myeloid leukaemia refractory to hypomethylating agents: a single-centre, single-arm, phase 2 trial.Lancet Haematol. 2020 Aug;7(8):e566-e574. doi: 10.1016/S2352-3026(20)30209-X. Lancet Haematol. 2020. PMID: 32735836 Free PMC article. Clinical Trial.
-
Development and manufacture of novel locally produced anti-BCMA CAR T cells for the treatment of relapsed/refractory multiple myeloma: results from a phase I clinical trial.Haematologica. 2023 Jul 1;108(7):1827-1839. doi: 10.3324/haematol.2022.281628. Haematologica. 2023. PMID: 36200421 Free PMC article. Clinical Trial.
-
How I treat a refractory myeloma patient who is not eligible for a clinical trial.Hematology Am Soc Hematol Educ Program. 2019 Dec 6;2019(1):125-136. doi: 10.1182/hematology.2019000016. Hematology Am Soc Hematol Educ Program. 2019. PMID: 31808850 Free PMC article.
-
PSPC1-interchanged interactions with PTK6 and β-catenin synergize oncogenic subcellular translocations and tumor progression.Nat Commun. 2019 Dec 16;10(1):5716. doi: 10.1038/s41467-019-13665-6. Nat Commun. 2019. PMID: 31844057 Free PMC article.
References
-
- Dimopoulos MA, Richardson PG, Moreau P, et al. : Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol 12:42-54, 2015 - PubMed
-
- Dimopoulos MA, Moreau P, Palumbo A, et al. : Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol 17:27-38, 2016 - PubMed
-
- San Miguel J, Weisel K, Moreau P, et al. : Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol 14:1055-1066, 2013 - PubMed
-
- Richardson P, Jagannath S, Hussein M, et al. : Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood 114:772-778, 2009 - PubMed
-
- Lonial S, Weiss BM, Usmani SZ, et al. : Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387:1551-1560, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

