Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- PMID: 29381702
- PMCID: PMC5790238
- DOI: 10.1371/journal.pmed.1002491
Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
Abstract
Background: Published estimates of mortality and progression to AIDS as children with HIV approach adulthood are limited. We describe rates and risk factors for death and AIDS-defining events in children and adolescents after initiation of combination antiretroviral therapy (cART) in 17 middle- and high-income countries, including some in Western and Central Europe (W&CE), Eastern Europe (Russia and Ukraine), and Thailand.
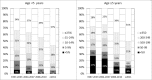
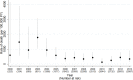
Methods and findings: Children with perinatal HIV aged <18 years initiating cART were followed until their 21st birthday, transfer to adult care, death, loss to follow-up, or last visit up until 31 December 2013. Rates of death and first AIDS-defining events were calculated. Baseline and time-updated risk factors for early/late (≤/>6 months of cART) death and progression to AIDS were assessed. Of 3,526 children included, 32% were from the United Kingdom or Ireland, 30% from elsewhere in W&CE, 18% from Russia or Ukraine, and 20% from Thailand. At cART initiation, median age was 5.2 (IQR 1.4-9.3) years; 35% of children aged <5 years had a CD4 lymphocyte percentage <15% in 1997-2003, which fell to 15% of children in 2011 onwards (p < 0.001). Similarly, 53% and 18% of children ≥5 years had a CD4 count <200 cells/mm3 in 1997-2003 and in 2011 onwards, respectively (p < 0.001). Median follow-up was 5.6 (2.9-8.7) years. Of 94 deaths and 237 first AIDS-defining events, 43 (46%) and 100 (42%) were within 6 months of initiating cART, respectively. Multivariable predictors of early death were: being in the first year of life; residence in Russia, Ukraine, or Thailand; AIDS at cART start; initiating cART on a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen; severe immune suppression; and low BMI-for-age z-score. Current severe immune suppression, low current BMI-for-age z-score, and current viral load >400 c/mL predicted late death. Predictors of early and late progression to AIDS were similar. Study limitations include incomplete recording of US Centers for Disease Control (CDC) disease stage B events and serious adverse events in some countries; events that were distributed over a long time period, and that we lacked power to analyse trends in patterns and causes of death over time.
Conclusions: In our study, 3,526 children and adolescents with perinatal HIV infection initiated antiretroviral therapy (ART) in countries in Europe and Thailand. We observed that over 40% of deaths occurred ≤6 months after cART initiation. Greater early mortality risk in infants, as compared to older children, and in Russia, Ukraine, or Thailand as compared to W&CE, raises concern. Current severe immune suppression, being underweight, and unsuppressed viral load were associated with a higher risk of death at >6 months after initiation of cART.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: EC is employed by UCL and currently receiving an MRC PhD studentship. LG received payments for conferences and participation in the scientific board from ViiV Healthcare. CT has participated in an advisory board for ViiV Healthcare and received research grants from the European Commission, UK Medical Research Council, Public Health England, PENTA Foundation, AbbVie, and ViiV. CT is on the advisory board of the Antiretroviral Pregnancy Registry, the Board of PENTA Foundation, and the UK Infectious Diseases in Pregnancy Screening Advisory Group. JW's academic institution received grants for the project and research from INSERM-ANRS (Agence Nationale de Recherche sur le sida et les hépatites virales, Agence autonome INSERM). JW's institution has in the past received funding from SFCE (Société Française des Cancers de l'Enfant), ANSM (Agence Nationale de Sécurité des édicaments et Produits de santé), ViiV HealthCare, and Parexel.
Figures

References
-
- Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. (2007) Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis 45: 918–924. doi: 10.1086/521167 - DOI - PubMed
-
- Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, et al. (2010) Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr 53: 86–94. doi: 10.1097/QAI.0b013e3181b9869f - DOI - PMC - PubMed
-
- Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, et al. (2011) Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986–2004). Clin Infect Dis 53: 1024–1034. doi: 10.1093/cid/cir641 - DOI - PMC - PubMed
-
- de Martino M, Tovo PA, Balducci M, Galli L, Gabiano C, Rezza G, et al. (2000) Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA 284: 190–197. - PubMed
-
- Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, Wasmuth JC, et al. (2012) All-cause mortality in treated HIV-infected adults with CD4 >/ = 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 41: 433–445. doi: 10.1093/ije/dyr164 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

