Mycobacterium leprae genomes from naturally infected nonhuman primates
- PMID: 29381722
- PMCID: PMC5790234
- DOI: 10.1371/journal.pntd.0006190
Mycobacterium leprae genomes from naturally infected nonhuman primates
Abstract
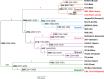
Leprosy is caused by the bacterial pathogens Mycobacterium leprae and Mycobacterium lepromatosis. Apart from humans, animals such as nine-banded armadillos in the Americas and red squirrels in the British Isles are naturally infected with M. leprae. Natural leprosy has also been reported in certain nonhuman primates, but it is not known whether these occurrences are due to incidental infections by human M. leprae strains or by M. leprae strains specific to nonhuman primates. In this study, complete M. leprae genomes from three naturally infected nonhuman primates (a chimpanzee from Sierra Leone, a sooty mangabey from West Africa, and a cynomolgus macaque from The Philippines) were sequenced. Phylogenetic analyses showed that the cynomolgus macaque M. leprae strain is most closely related to a human M. leprae strain from New Caledonia, whereas the chimpanzee and sooty mangabey M. leprae strains belong to a human M. leprae lineage commonly found in West Africa. Additionally, samples from ring-tailed lemurs from the Bezà Mahafaly Special Reserve, Madagascar, and chimpanzees from Ngogo, Kibale National Park, Uganda, were screened using quantitative PCR assays, to assess the prevalence of M. leprae in wild nonhuman primates. However, these samples did not show evidence of M. leprae infection. Overall, this study adds genomic data for nonhuman primate M. leprae strains to the existing M. leprae literature and finds that this pathogen can be transmitted from humans to nonhuman primates as well as between nonhuman primate species. While the prevalence of natural leprosy in nonhuman primates is likely low, nevertheless, future studies should continue to explore the prevalence of leprosy-causing pathogens in the wild.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Mycobacterium leprae and Mycobacterium lepraemurium infections in domestic and wild animals.Rev Sci Tech. 2001 Apr;20(1):219-51. doi: 10.20506/rst.20.1.1271. Rev Sci Tech. 2001. PMID: 11288514 Review.
-
Leprosy as a zoonosis: an update.Acta Leprol. 1988 Jan-Mar;6(1):51-60. Acta Leprol. 1988. PMID: 3051854 Review.
-
Leprosy in wild chimpanzees.Nature. 2021 Oct;598(7882):652-656. doi: 10.1038/s41586-021-03968-4. Epub 2021 Oct 13. Nature. 2021. PMID: 34646009 Free PMC article.
-
Spontaneous leprosy in a wild-caught cynomolgus macaque.Int J Lepr Other Mycobact Dis. 1998 Jun;66(2):140-8. Int J Lepr Other Mycobact Dis. 1998. PMID: 9728446
-
Differential growth of Mycobacterium leprae strains (SNP genotypes) in armadillos.Infect Genet Evol. 2018 Aug;62:20-26. doi: 10.1016/j.meegid.2018.04.017. Epub 2018 Apr 14. Infect Genet Evol. 2018. PMID: 29665434
Cited by
-
Lepra Bubalorum, a Potential Reservoir of Mycobacterium leprae.Front Microbiol. 2021 Dec 2;12:786921. doi: 10.3389/fmicb.2021.786921. eCollection 2021. Front Microbiol. 2021. PMID: 34925294 Free PMC article. Review.
-
2000-year-old pathogen genomes reconstructed from metagenomic analysis of Egyptian mummified individuals.BMC Biol. 2020 Aug 28;18(1):108. doi: 10.1186/s12915-020-00839-8. BMC Biol. 2020. PMID: 32859198 Free PMC article.
-
Armadillos and leprosy: from infection to biological model.Rev Inst Med Trop Sao Paulo. 2019 Sep 12;61:e44. doi: 10.1590/S1678-9946201961044. Rev Inst Med Trop Sao Paulo. 2019. PMID: 31531622 Free PMC article. Review.
-
Reservoirs and transmission routes of leprosy; A systematic review.PLoS Negl Trop Dis. 2020 Apr 27;14(4):e0008276. doi: 10.1371/journal.pntd.0008276. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32339201 Free PMC article.
-
Human macrophage polarization in the response to Mycobacterium leprae genomic DNA.Curr Res Microb Sci. 2020 Nov 26;2:100015. doi: 10.1016/j.crmicr.2020.100015. eCollection 2021 Dec. Curr Res Microb Sci. 2020. PMID: 34841308 Free PMC article.
References
-
- WHO. Global Leprosy Strategy 2016–2020. 2016.
-
- Britton WJ, Lockwood DNJ. Leprosy. Lancet. 2004;363(9416):1209–19. doi: 10.1016/S0140-6736(04)15952-7 - DOI - PubMed
-
- Gelber R. Leprosy (Hansen’s disease). Harrison’s Principles of Internal Medicine. 2005.
-
- Vargas-Ocampo F. Diffuse leprosy of Lucio and Latapí: A Histologic study. Lepr Rev. 2007;78(3):248–60. - PubMed
-
- Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130(6):856–64. doi: 10.1309/AJCPP72FJZZRRVMM - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

