Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer
- PMID: 29381935
- PMCID: PMC5708934
- DOI: 10.1097/MD.0000000000008635
Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer
Abstract
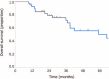
Although a few investigators have demonstrated the effect of concurrent chemoradiotherapy (CRT) for postoperative recurrent non-small cell lung cancer (NSCLC), the outcome of this treatment remains unclear. The aim of this study was to elucidate the efficacy and tolerability of concurrent CRT with cisplatin (CDDP) and vinorelbine (VNR) in patients with postoperative locoregional recurrent NSCLC. A total of 40 patients who had received concurrent CRT with CDDP and VNR between January 1999 and December 2014 were retrospectively analyzed. Patients were treated with CDDP (80 mg/m on day 1) and VNR (20 mg/m on days 1 and 8) every 4 weeks. Radiotherapy was administered concurrently during cycle 1. The delivered x-ray radiation dose was 60 Gy in all 37 patients who received x-ray radiotherapy; 3 patients received proton beam radiation (66 Gy [RBE] in 1 patient and 60 Gy [RBE] in 2 patients). The objective response rate was 85% (95% confidence interval [CI], 70.9%-92.9%). The median progression-free survival was 20.3 months (95% CI, 12.9 months-not reached). The 2-year survival rate was 78.9% (95% CI, 63.0%-89.1%). The most common grade ≥3 toxicity was neutropenia (18%). No grade ≥3 radiation pneumonitis and no treatment-related deaths were observed.Our study revealed that concurrent CRT with CDDP and VNR was active and safe for patients with postoperative locoregional recurrent NSCLC. Salvage CRT for postoperative locoregional recurrent NSCLC might be a promising treatment for selected patients.
Copyright © 2017 The Authors. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Williams BA, Sugimura H, Endo C, et al. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann Thorac Surg 2006;81:1021–7. - PubMed
-
- Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg 2007;83:409–17. - PubMed
-
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192–6. - PubMed
-
- Hung JJ, Jeng WJ, Hsu WH, et al. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012;7:1115–23. - PubMed
-
- Endo C, Sakurada A, Notsuda H, et al. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg 2012;93:1061–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

