Optimum end-tidal concentration of sevoflurane to facilitate supraglottic airway device insertion with propofol at induction allowing spontaneous respiration in obese patients: A prospective observational study
- PMID: 29382022
- PMCID: PMC5709021
- DOI: 10.1097/MD.0000000000008902
Optimum end-tidal concentration of sevoflurane to facilitate supraglottic airway device insertion with propofol at induction allowing spontaneous respiration in obese patients: A prospective observational study
Abstract
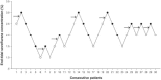
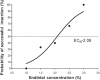
Obese patients are more likely to encounter with difficult airway management, and supraglottic airway device has been adopted to facilitate tracheal intubation. The optimum anesthetic concentration for obese patients to insert a supraglottic airway device with spontaneous respiration has not been investigated. This study was designed to determine the end-tidal concentration of sevoflurane that would provide acceptable condition for supraglottic airway device insertion with propofol at induction in obese patients without using neuromuscular blockade.Thirty elective obese patients [body mass index (BMI) 30-50 kg/m] scheduled for bariatric surgery were enrolled in this study. Sevoflurane was inhaled at a concentration of 5% after infusion of 1 mg/kg propofol (within 1 minute) according to lean body weight. The target concentration of sevoflurane was initiated at 2.5% with 0.5% as a step size using a modified Dixon up-and-down method. Five minutes after target concentration achieved, the insertion of supraglottic airway device was attempted.The minimum alveolar concentration of sevoflurane for successful insertion of supraglottic airway device calculated using up-and-down method were 2.25 (0.53) % for obese patients. The values of the effective concentration of sevoflurane for successful supraglottic airway device insertion in 50% (EC50) and 95% (EC95) of the obese patients obtained by probit regression analysis were 2.09% (95% confidence interval 1.48-2.68) and 3.31% (95% confidence interval 2.70-8.12), respectively.We conclude that sevoflurane at a minimum alveolar concentration of 2.25% can provide optimal conditions for insertion of supraglottic airway device with spontaneous respiration in obese patients with 1 mg/kg propofol at induction.
Copyright © 2017 The Authors. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

Similar articles
-
Dexmedetomidine reduces sevoflurane EC50 for supraglottic airway device insertion in spontaneously breathing morbidly obese patients.Ther Clin Risk Manag. 2019 May 3;15:627-635. doi: 10.2147/TCRM.S199440. eCollection 2019. Ther Clin Risk Manag. 2019. PMID: 31118650 Free PMC article.
-
The optimum sevoflurane concentration for supraglottic airway device Blockbuster™ insertion with spontaneous breathing in obese patients: a prospective observational study.BMC Anesthesiol. 2017 Nov 28;17(1):156. doi: 10.1186/s12871-017-0449-5. BMC Anesthesiol. 2017. PMID: 29179689 Free PMC article. Clinical Trial.
-
Optimum sevoflurane concentration for I-gel insertion in unpremedicated children.J Clin Anesth. 2015 Dec;27(8):627-31. doi: 10.1016/j.jclinane.2015.05.024. Epub 2015 Jul 7. J Clin Anesth. 2015. PMID: 26162594 Clinical Trial.
-
Supreme™ laryngeal mask airway insertion requires a lower concentration of sevoflurane than ProSeal™ laryngeal mask airway insertion during target-controlled remifentanil infusion: a prospective randomised controlled study.BMC Anesthesiol. 2020 Jan 7;20(1):5. doi: 10.1186/s12871-019-0921-5. BMC Anesthesiol. 2020. PMID: 31910822 Free PMC article. Clinical Trial.
-
Insertion of the cuffed oropharyngeal airway (COPA) with propofol or sevoflurane in adults.J Clin Anesth. 1999 Jun;11(4):280-4. doi: 10.1016/s0952-8180(99)00037-9. J Clin Anesth. 1999. PMID: 10470627 Clinical Trial.
Cited by
-
A feasibility study of jaw thrust as an indicator assessing adequate depth of anesthesia for insertion of supraglottic airway device in morbidly obese patients.Chin Med J (Engl). 2019 Sep 20;132(18):2185-2191. doi: 10.1097/CM9.0000000000000403. Chin Med J (Engl). 2019. PMID: 31425359 Free PMC article.
-
Dexmedetomidine reduces sevoflurane EC50 for supraglottic airway device insertion in spontaneously breathing morbidly obese patients.Ther Clin Risk Manag. 2019 May 3;15:627-635. doi: 10.2147/TCRM.S199440. eCollection 2019. Ther Clin Risk Manag. 2019. PMID: 31118650 Free PMC article.
-
Airway management for patients with tracheal stenosis and severe scar contracture of the face and neck via bronchoscopy: a case report.J Cardiothorac Surg. 2024 Sep 20;19(1):537. doi: 10.1186/s13019-024-03064-4. J Cardiothorac Surg. 2024. PMID: 39304900 Free PMC article.
References
-
- Murphy C, Wong DT. Airway management and oxygenation in obese patients. Can J Anaesth 2013;60:929–45. - PubMed
-
- Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013;119:1360–9. - PubMed
-
- Riad W, Vaez MN, Raveendran R, et al. Neck circumference as a predictor of difficult intubation and difficult mask ventilation in morbidly obese patients: a prospective observational study. Eur J Anaesthesiol 2016;33:244–9. - PubMed
-
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

