Botulinum Toxin A for Sialorrhoea Associated with Neurological Disorders: Evaluation of the Relationship between Effect of Treatment and the Number of Glands Treated
- PMID: 29382036
- PMCID: PMC5848156
- DOI: 10.3390/toxins10020055
Botulinum Toxin A for Sialorrhoea Associated with Neurological Disorders: Evaluation of the Relationship between Effect of Treatment and the Number of Glands Treated
Abstract
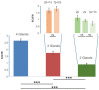
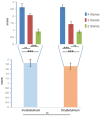
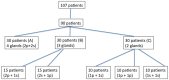
Background: Sialorrhoea and drooling are disabling manifestations of different neurological disorders. The aim of this study was to evaluate the effects of botulinum neurotoxin type A (BoNT/A) injection on hypersalivation in 90 patients with neurological diseases of different aetiologies, and to define the minimum number of injected salivary glands to reduce sialorrhoea. Determining the minimum number of glands that need to be engaged in order to have a significant reduction in drooling may be very useful for establishing the minimum total dosage of BoNT/A that may be considered effective in the treatment of hypersalivation. Methods: Twenty-five mouse units (MU) of BoNT/A (onabotulinumtoxin A, Botox; Allergan, Irvine, CA, USA; 100 MU/2 mL, 0.9% saline; or incobotulinumtoxin A, Xeomin; Merz Pharma, Germany; 100 MU/2 mL, 0.9% saline) were percutaneously injected into the parotid (p) glands and/or submandibular (s) glands under ultrasound control. On this basis, patients were divided into three groups. In group A (30 patients), BoNT/A injections were performed into four glands; in group B (30 patients), into three glands, and in group C (30 patients), into two glands. Patients treated in three glands (group B) were divided into two subgroups based on the treated glands (2 p + 1 s = 15 patients; 2 s + 1 p = 15 patients). Similarly, patients being injected in two glands (group C) were subdivided into three groups (2 p = 10 patients; 1 p + 1 s = 10 patients; 2 s = 10 patients). In patients who were injected in three and two salivary glands, saline solution was injected into the remaining one and two glands, respectively. Assessments were performed at baseline and at 2 weeks after the injections. Results: BoNT/A significantly reduced sialorrhoea in 82 out of 90 patients. The effect was more evident in patients who had four glands injected than when three or two glands were injected. The injections into three glands were more effective than injections into two glands. Conclusions: Our results have shown that BoNT/A injections induced a significant reduction in sialorrhoea in most patients (91%). In addition, we demonstrated that sialorrhoea associated with different neurological diseases was better controlled when the number of treated glands was higher.
Keywords: botulinum toxin; drooling; eccrine glands; incobotulinumtoxin A; onabotulinumtoxin A; salivary glands; sialorrhoea; swallowing.
Conflict of interest statement
The authors declare no conflict of interest. We had no founding sponsor.
Figures
Similar articles
-
Botulinum toxin injections for chronic sialorrhoea in children are effective regardless of the degree of neurological dysfunction: A single tertiary institution experience.Int J Pediatr Otorhinolaryngol. 2016 Sep;88:142-5. doi: 10.1016/j.ijporl.2016.06.031. Epub 2016 Jun 11. Int J Pediatr Otorhinolaryngol. 2016. PMID: 27497402
-
Botulinum Toxin in the Management of Sialorrhoea in Acquired Brain Injury.Ir Med J. 2016 Jun 10;109(6):425. Ir Med J. 2016. PMID: 27814442
-
Factors in the Efficacy, Safety, and Impact on Quality of Life for Treatment of Drooling with Botulinum Toxin Type A in Patients with Cerebral Palsy.Am J Phys Med Rehabil. 2017 Feb;96(2):68-76. doi: 10.1097/PHM.0000000000000525. Am J Phys Med Rehabil. 2017. PMID: 28099276 Clinical Trial.
-
Acute deterioration of bulbar function after botulinum toxin treatment for sialorrhoea in amyotrophic lateral sclerosis.Am J Phys Med Rehabil. 2008 Apr;87(4):321-4. doi: 10.1097/PHM.0b013e318164a931. Am J Phys Med Rehabil. 2008. PMID: 18303472 Review.
-
Comparing the evidence for botulinum neurotoxin injections in paediatric anterior drooling: a scoping review.Eur J Pediatr. 2024 Jan;183(1):83-93. doi: 10.1007/s00431-023-05309-1. Epub 2023 Nov 4. Eur J Pediatr. 2024. PMID: 37924348 Free PMC article.
Cited by
-
SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea.Neurology. 2019 Apr 23;92(17):e1982-e1991. doi: 10.1212/WNL.0000000000007368. Epub 2019 Mar 27. Neurology. 2019. PMID: 30918101 Free PMC article. Clinical Trial.
-
Cost-Effectiveness of IncobotulinumtoxinA in the Treatment of Sialorrhea in Patients with Various Neurological Conditions.Neurol Ther. 2020 Jun;9(1):117-133. doi: 10.1007/s40120-020-00182-8. Epub 2020 Mar 12. Neurol Ther. 2020. PMID: 32162214 Free PMC article.
-
Botulinum toxin in the treatment of sialorrhea in severe neurological patients with tracheotomy.Brain Behav. 2023 Aug;13(8):e3164. doi: 10.1002/brb3.3164. Epub 2023 Jul 17. Brain Behav. 2023. PMID: 37461166 Free PMC article.
-
Incobotulinumtoxin A for Sialorrhea in Neurological Disorders: A Real-Life Experience.Toxins (Basel). 2018 May 28;10(6):217. doi: 10.3390/toxins10060217. Toxins (Basel). 2018. PMID: 29843420 Free PMC article.
-
Submandibular Gland Reduction Using Botulinum Toxin Type A for a Smooth Jawline.Plast Reconstr Surg Glob Open. 2019 Apr 1;7(4):e2192. doi: 10.1097/GOX.0000000000002192. eCollection 2019 Apr. Plast Reconstr Surg Glob Open. 2019. PMID: 31321186 Free PMC article. No abstract available.
References
-
- Fuster Torres M.A., Berini Aytes L., Gay Escoda C. Salivary gland application of botulinum toxin for the treatment of sialorrhea. Med. Oral Patol. Oral Cir. Bucal. 2007;12:E511–E517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

