A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture
- PMID: 29382079
- PMCID: PMC5855607
- DOI: 10.3390/ijms19020385
A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture
Abstract
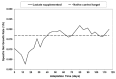
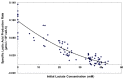
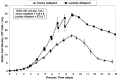
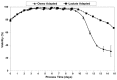
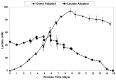
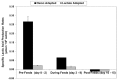
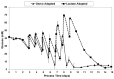
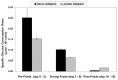
Fed-batch animal cell culture is the most common method for commercial production of recombinant proteins. However, higher cell densities in these platforms are still limited due to factors such as excessive ammonium production, lactic acid production, nutrient limitation, and/or hyperosmotic stress related to nutrient feeds and base additions to control pH. To partly overcome these factors, we investigated a simple method to reduce both ammonium and lactic acid production-termed Lactate Supplementation and Adaptation (LSA) technology-through the use of CHO cells adapted to a lactate-supplemented medium. Using this simple method, we achieved a reduction of nearly 100% in lactic acid production with a simultaneous 50% reduction in ammonium production in batch shaker flasks cultures. In subsequent fed-batch bioreactor cultures, lactic acid production and base addition were both reduced eight-fold. Viable cell densities of 35 million cells per mL and integral viable cell days of 273 million cell-days per mL were achieved, both among the highest currently reported for a fed-batch animal cell culture. Investigating the benefits of LSA technology in animal cell culture is worthy of further consideration and may lead to process conditions more favorable for advanced industrial applications.
Keywords: ammonium; animal cell culture; fed-batch; industrial; lactic acid; recombinant proteins.
Conflict of interest statement
The rights to LSA technology are currently held by Keck Graduate Institute. The authors are currently due a fraction of any licensing fees and/or royalties. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
Feed development for fed-batch CHO production process by semisteady state analysis.Biotechnol Prog. 2010 May-Jun;26(3):797-804. doi: 10.1002/btpr.362. Biotechnol Prog. 2010. PMID: 20014108
-
Utilization of tyrosine- and histidine-containing dipeptides to enhance productivity and culture viability.Biotechnol Bioeng. 2012 Sep;109(9):2286-94. doi: 10.1002/bit.24507. Epub 2012 Apr 8. Biotechnol Bioeng. 2012. PMID: 22447498
-
Concomitant reduction of lactate and ammonia accumulation in fed-batch cultures: Impact on glycoprotein production and quality.Biotechnol Prog. 2018 Mar;34(2):494-504. doi: 10.1002/btpr.2607. Epub 2018 Jan 18. Biotechnol Prog. 2018. PMID: 29314777
-
Bioreactor systems for the production of biopharmaceuticals from animal cells.Biotechnol Appl Biochem. 2006 Jul;45(Pt 1):1-12. doi: 10.1042/BA20050233. Biotechnol Appl Biochem. 2006. PMID: 16764553 Review.
-
Metabolic flux analysis in mammalian cell culture.Metab Eng. 2010 Mar;12(2):161-71. doi: 10.1016/j.ymben.2009.09.002. Epub 2009 Oct 13. Metab Eng. 2010. PMID: 19833223 Review.
Cited by
-
Characterization of metabolic responses, genetic variations, and microsatellite instability in ammonia-stressed CHO cells grown in fed-batch cultures.BMC Biotechnol. 2021 Jan 8;21(1):4. doi: 10.1186/s12896-020-00667-2. BMC Biotechnol. 2021. PMID: 33419422 Free PMC article.
-
Empirical economic analysis shows cost-effective continuous manufacturing of cultivated chicken using animal-free medium.Nat Food. 2024 Aug;5(8):693-702. doi: 10.1038/s43016-024-01022-w. Epub 2024 Aug 21. Nat Food. 2024. PMID: 39179871
-
Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges.Adv Sci (Weinh). 2022 Jan;9(3):e2102908. doi: 10.1002/advs.202102908. Epub 2021 Nov 16. Adv Sci (Weinh). 2022. PMID: 34786874 Free PMC article. Review.
-
Regenerative and Anti-Inflammatory Potential of Regularly Fed, Starved Cells and Extracellular Vesicles In Vivo.Cells. 2022 Aug 30;11(17):2696. doi: 10.3390/cells11172696. Cells. 2022. PMID: 36078106 Free PMC article.
-
Enabling Allogeneic T Cell-Based Therapies: Scalable Stirred-Tank Bioreactor Mediated Manufacturing.Front Med Technol. 2022 May 30;4:850565. doi: 10.3389/fmedt.2022.850565. eCollection 2022. Front Med Technol. 2022. PMID: 35707712 Free PMC article.
References
-
- Leibovitz A. The growth and maintenance of tissue-cell cultures in free gas exchange with the atmosphere. Am. J. Hyg. 1963;78:173–180. - PubMed
-
- Fleischaker R.J. Ph.D. Thesis. Massachusetts Institute of Technology; Cambridge, MA, USA: Jun, 1982. An Experimental Study in the use of Instrumentation to Analyze Metabolism and Product Formation in Cell Culture.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

