Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies
- PMID: 29382085
- PMCID: PMC5855609
- DOI: 10.3390/ijms19020387
Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies
Abstract
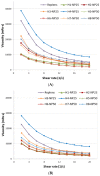
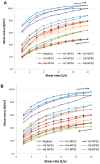
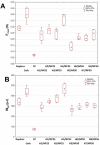
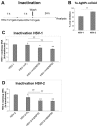
Mucoadhesive gelling systems with tannic acid modified silver nanoparticles were developed for effective treatment of herpes virus infections. To increase nanoparticle residence time after local application, semi solid formulations designed from generally regarded as safe (GRAS) excipients were investigated for their rheological and mechanical properties followed with ex vivo mucoadhesive behavior to the porcine vaginal mucosa. Particular effort was made to evaluate the activity of nanoparticle-based hydrogels toward herpes simplex virus (HSV) type 1 and 2 infection in vitro in immortal human keratinocyte cell line and in vivo using murine model of HSV-2 genital infection. The effect of infectivity was determined by real time quantitative polymerase chain reaction, plaque assay, inactivation, attachment, penetration and cell-to-cell assessments. All analyzed nanoparticle-based hydrogels exhibited pseudoplastic and thixotropic properties. Viscosity and mechanical measurements of hydrogels were found to correlate with the mucoadhesive properties. The results confirmed the ability of nanoparticle-based hydrogels to affect viral attachment, impede penetration and cell-to-cell transmission, although profound differences in the activity evoked by tested preparations toward HSV-1 and HSV-2 were noted. In addition, these findings demonstrated the in vivo potential of tannic acid modified silver nanoparticle-based hydrogels for vaginal treatment of HSV-2 genital infection.
Keywords: Carbopol 974P; HSV 1/2; antiherpes activity; hydrogel; mucoadhesiveness; tannic acid modified silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis.Viruses. 2018 Sep 26;10(10):524. doi: 10.3390/v10100524. Viruses. 2018. PMID: 30261662 Free PMC article.
-
Adjuvanticity of Tannic Acid-Modified Nanoparticles Improves Effectiveness of the Antiviral Response.Int J Nanomedicine. 2025 Apr 1;20:3977-3997. doi: 10.2147/IJN.S512509. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40191045 Free PMC article.
-
Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection.PLoS One. 2014 Aug 12;9(8):e104113. doi: 10.1371/journal.pone.0104113. eCollection 2014. PLoS One. 2014. PMID: 25117537 Free PMC article.
-
An overview application of silver nanoparticles in inhibition of herpes simplex virus.Artif Cells Nanomed Biotechnol. 2018 Mar;46(2):263-267. doi: 10.1080/21691401.2017.1307208. Epub 2017 Apr 12. Artif Cells Nanomed Biotechnol. 2018. PMID: 28403676 Review.
-
Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview.Viruses. 2018 Sep 3;10(9):473. doi: 10.3390/v10090473. Viruses. 2018. PMID: 30177661 Free PMC article. Review.
Cited by
-
Biocidal Activity of Tannic Acid-Prepared Silver Nanoparticles towards Pathogens Isolated from Patients with Exacerbations of Chronic Rhinosinusitis.Int J Mol Sci. 2022 Dec 6;23(23):15411. doi: 10.3390/ijms232315411. Int J Mol Sci. 2022. PMID: 36499763 Free PMC article.
-
Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms.Int J Mol Sci. 2022 Jan 21;23(3):1165. doi: 10.3390/ijms23031165. Int J Mol Sci. 2022. PMID: 35163090 Free PMC article. Review.
-
Silver Nanoparticle-Mediated Antiviral Efficacy against Enveloped Viruses: A Comprehensive Review.Glob Chall. 2025 Mar 28;9(5):2400380. doi: 10.1002/gch2.202400380. eCollection 2025 May. Glob Chall. 2025. PMID: 40352632 Free PMC article. Review.
-
Metabolomics-directed nanotechnology in viral diseases management: COVID-19 a case study.Pharmacol Rep. 2023 Oct;75(5):1045-1065. doi: 10.1007/s43440-023-00517-w. Epub 2023 Aug 16. Pharmacol Rep. 2023. PMID: 37587394 Free PMC article. Review.
-
Nanoparticles and Other Nanostructures and the Control of Pathogens: From Bench to Vaccines.Int J Mol Sci. 2023 May 21;24(10):9063. doi: 10.3390/ijms24109063. Int J Mol Sci. 2023. PMID: 37240409 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

