Activation of Aflatoxin Biosynthesis Alleviates Total ROS in Aspergillus parasiticus
- PMID: 29382166
- PMCID: PMC5848158
- DOI: 10.3390/toxins10020057
Activation of Aflatoxin Biosynthesis Alleviates Total ROS in Aspergillus parasiticus
Abstract
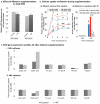
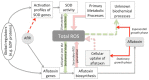
An aspect of mycotoxin biosynthesis that remains unclear is its relationship with the cellular management of reactive oxygen species (ROS). Here we conduct a comparative study of the total ROS production in the wild-type strain (SU-1) of the plant pathogen and aflatoxin producer, Aspergillus parasiticus, and its mutant strain, AFS10, in which the aflatoxin biosynthesis pathway is blocked by disruption of its pathway regulator, aflR. We show that SU-1 demonstrates a significantly faster decrease in total ROS than AFS10 between 24 h to 48 h, a time window within which aflatoxin synthesis is activated and reaches peak levels in SU-1. The impact of aflatoxin synthesis in alleviation of ROS correlated well with the transcriptional activation of five superoxide dismutases (SOD), a group of enzymes that protect cells from elevated levels of a class of ROS, the superoxide radicals (O₂-). Finally, we show that aflatoxin supplementation to AFS10 growth medium results in a significant reduction of total ROS only in 24 h cultures, without resulting in significant changes in SOD gene expression. Our findings show that the activation of aflatoxin biosynthesis in A. parasiticus alleviates ROS generation, which in turn, can be both aflR dependent and aflatoxin dependent.
Keywords: Aspergillus; Key Contribution; This work illustrates how aflatoxin biosynthesis contributes to the management of total ROS in Aspergillus parasiticus; aflR; aflatoxin; aflatoxin biosynthesis; an established model for studying mycotoxin biosynthesis and secondary metabolism in filamentous fungi. We show that activation of aflatoxin biosynthesis reduces total ROS production in this fungus; and aflatoxin itself.; is jointly mediated by the aflatoxin pathway regulator; reactive oxygen species; superoxide dismutase; which at least in part.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Aflatoxin biosynthesis is a novel source of reactive oxygen species--a potential redox signal to initiate resistance to oxidative stress?Toxins (Basel). 2015 Apr 28;7(5):1411-30. doi: 10.3390/toxins7051411. Toxins (Basel). 2015. PMID: 25928133 Free PMC article.
-
Pre-termination in aflR of Aspergillus sojae inhibits aflatoxin biosynthesis.Appl Microbiol Biotechnol. 2001 May;55(5):585-9. doi: 10.1007/s002530100607. Appl Microbiol Biotechnol. 2001. PMID: 11414325
-
Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae.J Biotechnol. 2004 Feb 5;107(3):245-53. doi: 10.1016/j.jbiotec.2003.10.012. J Biotechnol. 2004. PMID: 14736460
-
Understanding nonaflatoxigenicity of Aspergillus sojae: a windfall of aflatoxin biosynthesis research.Appl Microbiol Biotechnol. 2007 Oct;76(5):977-84. doi: 10.1007/s00253-007-1116-4. Epub 2007 Jul 31. Appl Microbiol Biotechnol. 2007. PMID: 17665189 Review.
-
Genetic regulation of aflatoxin biosynthesis: from gene to genome.Fungal Genet Biol. 2009 Feb;46(2):113-25. doi: 10.1016/j.fgb.2008.10.011. Epub 2008 Nov 5. Fungal Genet Biol. 2009. PMID: 19010433 Review.
Cited by
-
Analysis of the Relationship between Alternative Respiration and Sterigmatocystin Formation in Aspergillus nidulans.Toxins (Basel). 2018 Apr 20;10(4):168. doi: 10.3390/toxins10040168. Toxins (Basel). 2018. PMID: 29677138 Free PMC article.
-
The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize.Toxins (Basel). 2020 Sep 1;12(9):562. doi: 10.3390/toxins12090562. Toxins (Basel). 2020. PMID: 32882838 Free PMC article.
-
Enhancement of Pneumocandin B0 Production in Glarea lozoyensis by Low-Temperature Adaptive Laboratory Evolution.Front Microbiol. 2018 Nov 21;9:2788. doi: 10.3389/fmicb.2018.02788. eCollection 2018. Front Microbiol. 2018. PMID: 30519220 Free PMC article.
-
The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation.Front Plant Sci. 2022 Dec 12;13:1044896. doi: 10.3389/fpls.2022.1044896. eCollection 2022. Front Plant Sci. 2022. PMID: 36578344 Free PMC article. Review.
-
Genome Analysis of the Broad Host Range Necrotroph Nalanthamala psidii Highlights Genes Associated With Virulence.Front Plant Sci. 2022 Feb 25;13:811152. doi: 10.3389/fpls.2022.811152. eCollection 2022. Front Plant Sci. 2022. PMID: 35283890 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

