Systemic sclerosis associated interstitial lung disease - individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group
- PMID: 29382380
- PMCID: PMC5791165
- DOI: 10.1186/s13075-018-1517-z
Systemic sclerosis associated interstitial lung disease - individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group
Abstract
Background: Interstitial lung disease in systemic sclerosis (SSc-ILD) is a major cause of SSc-related death. Imunosuppressive treatment (IS) is used in patients with SSc for various organ manifestations mainly to ameliorate progression of SSc-ILD. Data on everyday IS prescription patterns and clinical courses of lung function during and after therapy are scarce.
Methods: We analysed patients fulfilling American College of Rheumatology (ACR)/European League against Rheumatism (EULAR) 2013 criteria for SSc-ILD and at least one report of IS. Types of IS, pulmonary function tests (PFT) and PFT courses during IS treatment were evaluated.
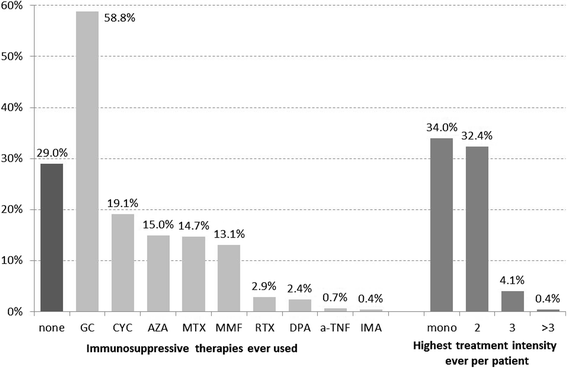
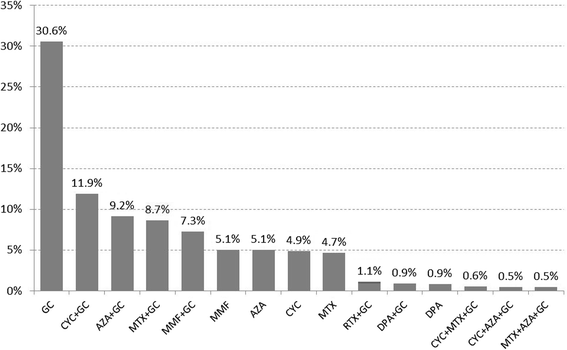
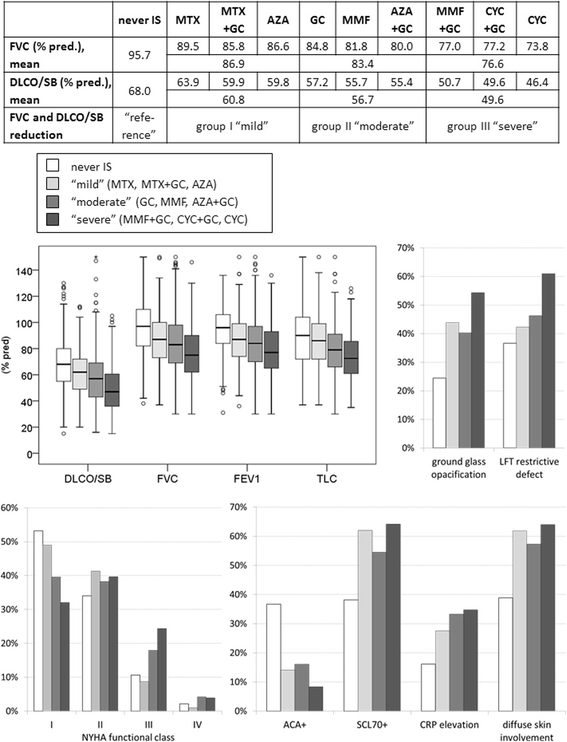
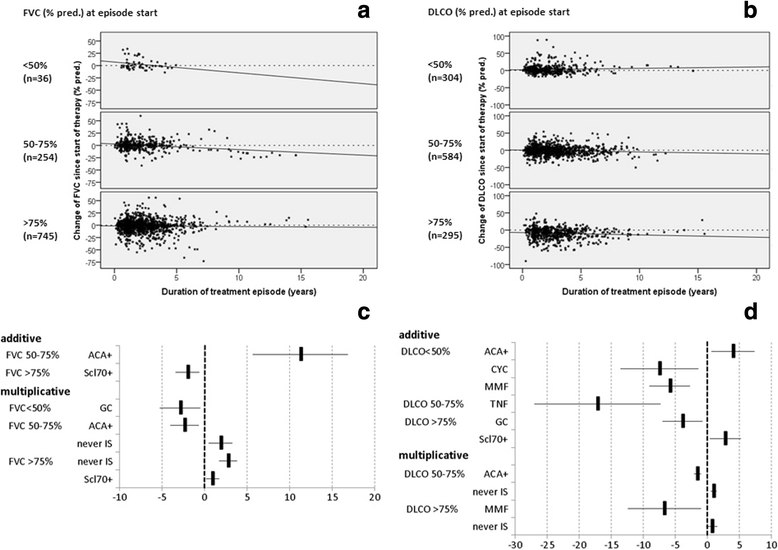
Results: EUSTAR contains 3778/11,496 patients with SSc-ILD (33%), with IS in 2681/3,778 (71%). Glucocorticoid (GC) monotherapy was prescribed in 30.6% patients with GC combinations plus cyclophosphamide (CYC) (11.9%), azathioprine (AZA) (9.2%), methotrexate (MTX) (8.7%), or mycophenolate mofetil (MMF) (7.3%). Intensive IS (MMF + GC, CYC or CYC + GC) was started in patients with the worst PFTs and ground glass opacifications on imaging. Patients without IS showed slightly less worsening in forced vital capacity (FVC) when starting with FVC 50-75% or >75%. GC showed negative trends when starting with FVC <50%. Regarding diffusing capacity for carbon monoxide (DLCO), negative DLCO trends were found in patients with MMF.
Conclusions: IS is broadly prescribed in SSc-ILD. Clusters of clinical and functional characteristics guide individualised treatment. Data favour distinguished decision-making, pointing to either watchful waiting and close monitoring in the early stages or start of immunosuppressive treatment in moderately impaired lung function. Advantages of specific IS are difficult to depict due to confounding by indication. Data do not support liberal use of GC in SSc-ILD.
Keywords: Follow up; Immunosuppressants; Interstitial lung disease; Lung function; Systemic sclerosis.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Khanna D, Tseng CH, Farmani N, Steen V, Furst DE, Clements PJ, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum. 2011;63(10):3078–85. doi: 10.1002/art.30467. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

