Pharmacokinetics of meropenem in septic patients on sustained low-efficiency dialysis: a population pharmacokinetic study
- PMID: 29382394
- PMCID: PMC5791175
- DOI: 10.1186/s13054-018-1940-1
Pharmacokinetics of meropenem in septic patients on sustained low-efficiency dialysis: a population pharmacokinetic study
Abstract
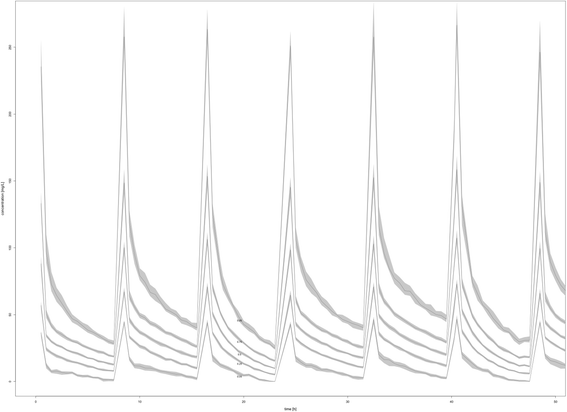
Background: The aim of the study was to describe the population pharmacokinetics (PK) of meropenem in critically ill patients receiving sustained low-efficiency dialysis (SLED).
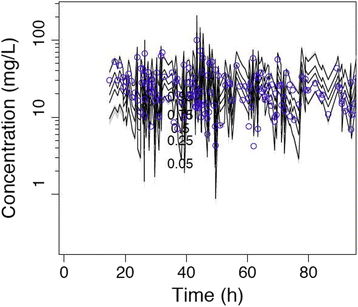
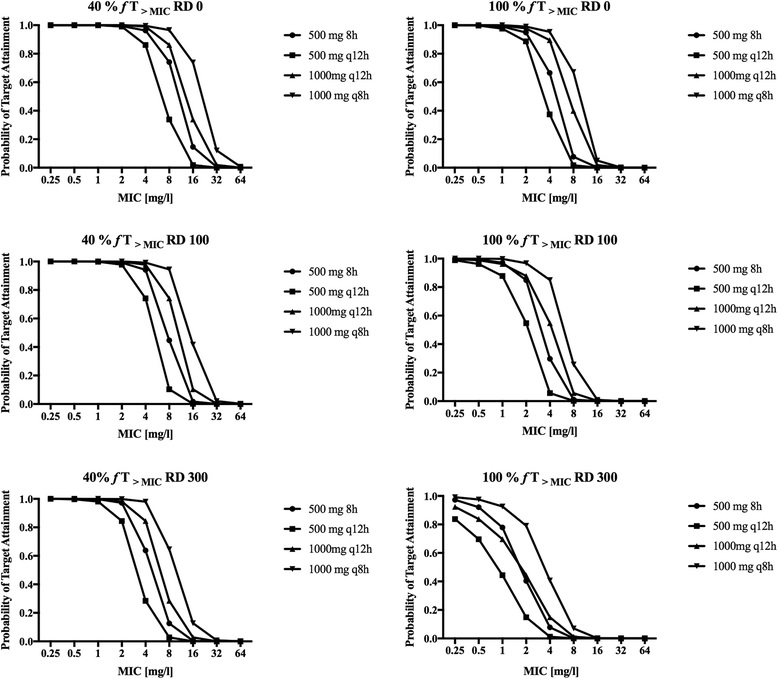
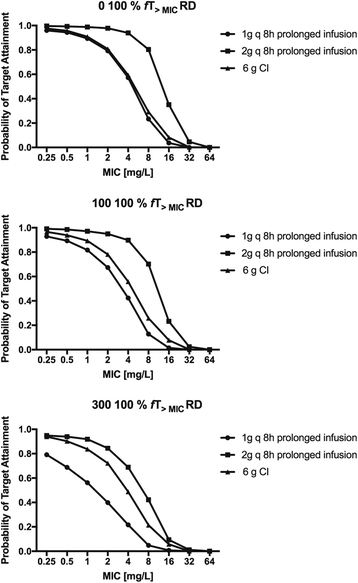
Methods: Prospective population PK study on 19 septic patients treated with meropenem and receiving SLED for acute kidney injury. Serial blood samples for determination of meropenem concentrations were taken before, during and after SLED in up to three sessions per patient. Nonparametric population PK analysis with Monte Carlo simulations were used. Pharmacodynamic (PD) targets of 40% and 100% time above the minimal inhibitory concentration (f T > MIC) were used for probability of target attainment (PTA) and fractional target attainment (FTA) against Pseudomonas aeruginosa.
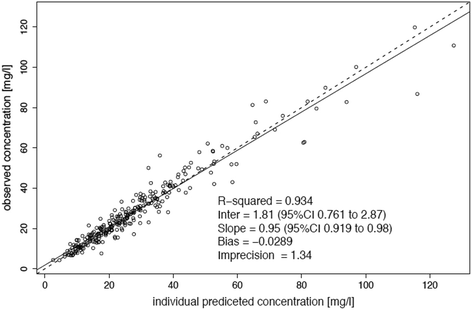
Results: A two-compartment linear population PK model was most appropriate with residual diuresis supported as significant covariate affecting meropenem clearance. In patients without residual diuresis the PTA for both targets (40% and 100% f T > MIC) and susceptible P. aeruginosa (MIC ≤ 2 mg/L) was > 95% for a dose of 0.5 g 8-hourly. In patients with a residual diuresis of 300 mL/d 1 g 12-hourly and 2 g 8-hourly would be required to achieve a PTA of > 95% and 93% for targets of 40% f T > MIC and 100% f T > MIC, respectively. A dose of 2 g 8-hourly would be able to achieve a FTA of 97% for 100% f T > MIC in patients with residual diuresis.
Conclusions: We found a relevant PK variability for meropenem in patients on SLED, which was significantly influenced by the degree of residual diuresis. As a result dosing recommendations for meropenem in patients on SLED to achieve adequate PD targets greatly vary. Therapeutic drug monitoring may help to further optimise individual dosing.
Trial registration: Clincialtrials.gov, NCT02287493 .
Keywords: Acute renal failure; Meropenem; Pharmacokinetics; Sepsis; Sustained low-efficiency dialysis.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the local ethics committee. Written informed consent was obtained either from the patient or their appointed legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Population pharmacokinetics and dosing simulations of ceftazidime in critically ill patients receiving sustained low-efficiency dialysis.J Antimicrob Chemother. 2017 May 1;72(5):1433-1440. doi: 10.1093/jac/dkw592. J Antimicrob Chemother. 2017. PMID: 28175308
-
Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units.Antimicrob Agents Chemother. 2015;59(6):2995-3001. doi: 10.1128/AAC.04166-14. Epub 2015 Mar 9. Antimicrob Agents Chemother. 2015. PMID: 25753628 Free PMC article.
-
Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements.Antimicrob Agents Chemother. 2015 Sep;59(9):5520-8. doi: 10.1128/AAC.00712-15. Epub 2015 Jun 29. Antimicrob Agents Chemother. 2015. PMID: 26124172 Free PMC article.
-
Optimal Meropenem Dosing Regimens in Patients Undergoing Continuous Renal Replacement Therapy: Systematic Review and Monte Carlo Simulations.Blood Purif. 2023;52(6):503-515. doi: 10.1159/000529694. Epub 2023 May 5. Blood Purif. 2023. PMID: 37231811
-
Antibiotic Dosing in Continuous Renal Replacement Therapy.Adv Chronic Kidney Dis. 2017 Jul;24(4):219-227. doi: 10.1053/j.ackd.2017.05.004. Adv Chronic Kidney Dis. 2017. PMID: 28778361 Review.
Cited by
-
Model-informed identification of optimised dosing strategies for meropenem in critically ill patients receiving SLEDD: an observational study.Infection. 2025 Apr 25. doi: 10.1007/s15010-025-02504-0. Online ahead of print. Infection. 2025. PMID: 40279026
-
Antibiotic Dosing in Sustained Low-Efficiency Dialysis in Critically Ill Patients.Can J Kidney Health Dis. 2018 Aug 10;5:2054358118792229. doi: 10.1177/2054358118792229. eCollection 2018. Can J Kidney Health Dis. 2018. PMID: 30116545 Free PMC article. Review.
-
Acute-on-chronic liver failure alters meropenem pharmacokinetics in critically ill patients with continuous hemodialysis: an observational study.Ann Intensive Care. 2020 Apr 22;10(1):48. doi: 10.1186/s13613-020-00666-8. Ann Intensive Care. 2020. PMID: 32323030 Free PMC article.
-
Meropenem and piperacillin/tazobactam optimised dosing regimens for critically ill patients receiving renal replacement therapy.Intensive Care Med. 2025 Aug 13. doi: 10.1007/s00134-025-08067-w. Online ahead of print. Intensive Care Med. 2025. PMID: 40801954
-
What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit?Pharmaceutics. 2020 Jul 7;12(7):638. doi: 10.3390/pharmaceutics12070638. Pharmaceutics. 2020. PMID: 32645953 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

