Mast Cell-Dependent CD8+ T-cell Recruitment Mediates Immune Surveillance of Intestinal Tumors in ApcMin/+ Mice
- PMID: 29382671
- PMCID: PMC9580996
- DOI: 10.1158/2326-6066.CIR-17-0424
Mast Cell-Dependent CD8+ T-cell Recruitment Mediates Immune Surveillance of Intestinal Tumors in ApcMin/+ Mice
Abstract
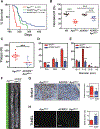
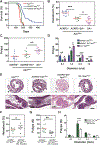
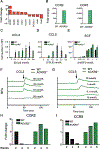
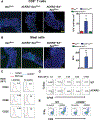
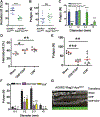
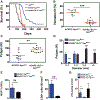
The presence of mast cells in some human colorectal cancers is a positive prognostic factor, but the basis for this association is incompletely understood. Here, we found that mice with a heterozygous mutation in the adenomatous polyposis coli gene (ApcMin/+) displayed reduced intestinal tumor burdens and increased survival in a chemokine decoy receptor, ACKR2-null background, which led to discovery of a critical role for mast cells in tumor defense. ACKR2-/-ApcMin/+ tumors showed increased infiltration of mast cells, their survival advantage was lost in mast cell-deficient ACKR2-/-SA-/-ApcMin/+ mice as the tumors grew rapidly, and adoptive transfer of mast cells restored control of tumor growth. Mast cells from ACKR2-/- mice showed elevated CCR2 and CCR5 expression and were also efficient in antigen presentation and activation of CD8+ T cells. Mast cell-derived leukotriene B4 (LTB4) was found to be required for CD8+ T lymphocyte recruitment, as mice lacking the LTB4 receptor (ACKR2-/-BLT1-/-ApcMin/+) were highly susceptible to intestinal tumor-induced mortality. Taken together, these data demonstrate that chemokine-mediated recruitment of mast cells is essential for initiating LTB4/BLT1-regulated CD8+ T-cell homing and generation of effective antitumor immunity against intestinal tumors. We speculate that the pathway reported here underlies the positive prognostic significance of mast cells in selected human tumors. Cancer Immunol Res; 6(3); 332-47. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
A. Mantovani is a consultant/advisory board member for Verily, Efranat, AbbVie, Compugen, Novartis, Pierre Fabre, and Roche. No potential conflicts of interest were disclosed by the other authors.
Figures


Similar articles
-
The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness.J Immunol. 2006 Mar 1;176(5):3157-64. doi: 10.4049/jimmunol.176.5.3157. J Immunol. 2006. PMID: 16493075
-
Leukotriene B₄-leukotriene B₄ receptor axis promotes oxazolone-induced contact dermatitis by directing skin homing of neutrophils and CD8⁺ T cells.Immunology. 2015 Sep;146(1):50-8. doi: 10.1111/imm.12478. Epub 2015 Jun 29. Immunology. 2015. PMID: 25959240 Free PMC article.
-
Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4.Nat Immunol. 2003 Oct;4(10):974-81. doi: 10.1038/ni971. Epub 2003 Aug 31. Nat Immunol. 2003. PMID: 12949532
-
The yin and yang of leukotriene B4 mediated inflammation in cancer.Semin Immunol. 2017 Oct;33:58-64. doi: 10.1016/j.smim.2017.09.005. Epub 2017 Oct 2. Semin Immunol. 2017. PMID: 28982616 Free PMC article. Review.
-
The role of leukotriene B(4) in allergic diseases.Allergol Int. 2008 Dec;57(4):291-8. doi: 10.2332/allergolint.08-RAI-0019. Epub 2008 Dec 1. Allergol Int. 2008. PMID: 18797182 Review.
Cited by
-
Regulatory T cells specifically suppress conventional CD8αβ T cells in intestinal tumors of APCMin/+ mice.Cancer Immunol Immunother. 2020 Jul;69(7):1279-1292. doi: 10.1007/s00262-020-02540-9. Epub 2020 Mar 17. Cancer Immunol Immunother. 2020. PMID: 32185408 Free PMC article.
-
Mast cells boost anti-tumor potency of MAIT cells via inflammasome-dependent secretion of IL-18.Nat Commun. 2025 Jul 2;16(1):6074. doi: 10.1038/s41467-025-61324-w. Nat Commun. 2025. PMID: 40603850 Free PMC article.
-
Mast cells: a double-edged sword in inflammation and fibrosis.Front Cell Dev Biol. 2024 Sep 17;12:1466491. doi: 10.3389/fcell.2024.1466491. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39355120 Free PMC article. Review.
-
Control of Cytoskeletal Dynamics by β-Arrestin1/Myosin Vb Signaling Regulates Endosomal Sorting and Scavenging Activity of the Atypical Chemokine Receptor ACKR2.Vaccines (Basel). 2020 Sep 17;8(3):542. doi: 10.3390/vaccines8030542. Vaccines (Basel). 2020. PMID: 32957704 Free PMC article.
-
Leukotriene B4 promotes neovascularization and macrophage recruitment in murine wet-type AMD models.JCI Insight. 2018 Sep 20;3(18):e96902. doi: 10.1172/jci.insight.96902. eCollection 2018 Sep 20. JCI Insight. 2018. PMID: 30232269 Free PMC article.
References
-
- Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res 2013;1:269–79. - PubMed
-
- Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta 2012;1822:2–8. - PubMed
-
- Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol 2009;24:1265–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

