Plant Temperature Acclimation and Growth Rely on Cytosolic Ribosome Biogenesis Factor Homologs
- PMID: 29382692
- PMCID: PMC5841729
- DOI: 10.1104/pp.17.01448
Plant Temperature Acclimation and Growth Rely on Cytosolic Ribosome Biogenesis Factor Homologs
Abstract
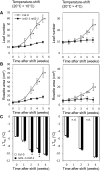
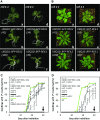
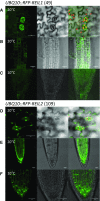
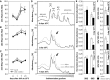
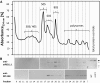
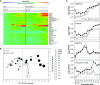
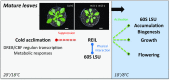
Arabidopsis (Arabidopsis thaliana) REI1-LIKE (REIL) proteins, REIL1 and REIL2, are homologs of a yeast ribosome biogenesis factor that participates in late cytoplasmic 60S ribosomal subunit maturation. Here, we report that the inhibited growth of the reil1-1 reil2-1 mutant at 10°C can be rescued by the expression of amino-terminal FLUORESCENT PROTEIN (FP)-REIL fusions driven by the UBIQUITIN10 promoter, allowing the analysis of REIL function in planta. Arabidopsis REIL1 appears to be functionally conserved, based on the cytosolic localization of FP-REIL1 and the interaction of native REIL1 with the 60S subunit in wild-type plants. In contrast to its yeast homologs, REIL1 also was present in translating ribosome fractions. Systems analysis revealed that wild-type Arabidopsis remodels the cytosolic translation machinery when grown at 10°C by accumulating cytosolic ribosome subunits and inducing the expression of cytosolic ribosomal RNA, ribosomal genes, ribosome biogenesis factors, and translation initiation or elongation factors. In the reil1-1 reil2-1 mutant, all processes associated with inhibited growth were delayed, although the plants maintained cellular integrity or acquired freezing tolerance. REIL proteins also were implicated in plant-specific processes: nonacclimated reil1-1 reil2-1 exhibited cold-acclimation responses, including activation of the DREB/CBF regulon. In addition, acclimated reil1-1 reil2-1 plants failed to activate FLOWERING LOCUS T expression in mature leaves. Therefore, in the wild type, REIL function may contribute to temperature perception by suppressing premature cold responses during growth at nonstressful temperatures. In conclusion, we suggest that Arabidopsis REIL proteins influence cold-induced plant ribosome remodeling and enhance the accumulation of cytosolic ribosome subunits after cold shift either by de novo synthesis or by recycling them from the translating ribosome fraction.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures





Similar articles
-
The REIL1 and REIL2 proteins of Arabidopsis thaliana are required for leaf growth in the cold.Plant Physiol. 2013 Dec;163(4):1623-39. doi: 10.1104/pp.113.223925. Epub 2013 Sep 13. Plant Physiol. 2013. PMID: 24038679 Free PMC article.
-
Arabidopsis REI-LIKE proteins activate ribosome biogenesis during cold acclimation.Sci Rep. 2021 Jan 28;11(1):2410. doi: 10.1038/s41598-021-81610-z. Sci Rep. 2021. PMID: 33510206 Free PMC article.
-
REIL proteins of Arabidopsis thaliana interact in yeast-2-hybrid assays with homologs of the yeast Rlp24, Rpl24A, Rlp24B, Arx1, and Jjj1 proteins.Plant Signal Behav. 2014;9(3):e28224. doi: 10.4161/psb.28224. Epub 2014 Mar 6. Plant Signal Behav. 2014. PMID: 24603461 Free PMC article.
-
Spatially Enriched Paralog Rearrangements Argue Functionally Diverse Ribosomes Arise during Cold Acclimation in Arabidopsis.Int J Mol Sci. 2021 Jun 7;22(11):6160. doi: 10.3390/ijms22116160. Int J Mol Sci. 2021. PMID: 34200446 Free PMC article.
-
Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana.Plant Cell Rep. 2019 May;38(5):511-519. doi: 10.1007/s00299-019-02376-3. Epub 2019 Jan 16. Plant Cell Rep. 2019. PMID: 30652229 Free PMC article. Review.
Cited by
-
Systematic Review of Plant Ribosome Heterogeneity and Specialization.Front Plant Sci. 2020 Jun 25;11:948. doi: 10.3389/fpls.2020.00948. eCollection 2020. Front Plant Sci. 2020. PMID: 32670337 Free PMC article.
-
Differential Morpho-Physiological and Transcriptomic Responses to Heat Stress in Two Blueberry Species.Int J Mol Sci. 2021 Mar 1;22(5):2481. doi: 10.3390/ijms22052481. Int J Mol Sci. 2021. PMID: 33804571 Free PMC article.
-
Seven plant capacities to adapt to abiotic stress.J Exp Bot. 2023 Aug 17;74(15):4308-4323. doi: 10.1093/jxb/erad179. J Exp Bot. 2023. PMID: 37220077 Free PMC article. Review.
-
Multi-Approach Analysis Reveals Pathways of Cold Tolerance Divergence in Camellia japonica.Front Plant Sci. 2022 Feb 25;13:811791. doi: 10.3389/fpls.2022.811791. eCollection 2022. Front Plant Sci. 2022. PMID: 35283896 Free PMC article.
-
Transcriptomic analysis of Stropharia rugosoannulata reveals carbohydrate metabolism and cold resistance mechanisms under low-temperature stress.AMB Express. 2022 May 14;12(1):56. doi: 10.1186/s13568-022-01400-2. AMB Express. 2022. PMID: 35567721 Free PMC article.
References
-
- Ascenzi R, Gantt JS (1999) Molecular genetic analysis of the drought-inducible linker histone variant in Arabidopsis thaliana. Plant Mol Biol 41: 159–169 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

