Age-Dependent Decline in Fate Switch from NG2 Cells to Astrocytes After Olig2 Deletion
- PMID: 29382710
- PMCID: PMC5830521
- DOI: 10.1523/JNEUROSCI.0712-17.2018
Age-Dependent Decline in Fate Switch from NG2 Cells to Astrocytes After Olig2 Deletion
Abstract
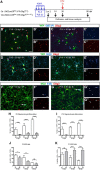
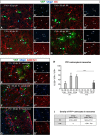
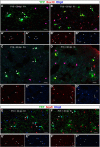
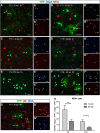
NG2 cells are a resident glial progenitor cell population that is uniformly distributed throughout the developing and mature mammalian CNS. Those in the postnatal CNS generate exclusively myelinating and non-myelinating oligodendrocytes and are thus equated with oligodendrocyte precursor cells. Prenatally, NG2 cells in the ventral gray matter of the forebrain generate protoplasmic astrocytes as well as oligodendrocytes. The fate conversion from NG2 cells into protoplasmic astrocytes is dependent on downregulation of the key oligodendrocyte transcription factor Olig2. We showed previously that constitutive deletion of Olig2 in NG2 cells converts NG2 cells in the neocortex into protoplasmic astrocytes at the expense of oligodendrocytes. In this study, we show that postnatal deletion of Olig2 caused NG2 cells in the neocortex but not in other gray matter regions to become protoplasmic astrocytes. However, NG2 cells in the neocortex became more resistant to astrocyte fate switch over the first 3 postnatal weeks. Fewer NG2 cells differentiated into astrocytes and did so with longer latency after Olig2 deletion at postnatal day 18 (P18) compared with deletion at P2. The high-mobility group transcription factor Sox10 was not downregulated for at least 1 month after Olig2 deletion at P18 despite an early transient upregulation of the astrocyte transcription factor NFIA. Furthermore, inhibiting cell proliferation in slice culture reduced astrocyte differentiation from Olig2-deleted perinatal NG2 cells, suggesting that cell division might facilitate nuclear reorganization needed for astrocyte transformation.SIGNIFICANCE STATEMENT NG2 cells are glial progenitor cells that retain a certain degree of lineage plasticity. In the normal postnatal neocortex, they generate mostly oligodendrocyte lineage cells. When the oligodendrocyte transcription factor Olig2 is deleted in NG2 cells in the neocortex, they switch their fate to protoplasmic astrocytes. However, the efficiency of the fate switch decreases with age over the first 3 postnatal weeks and is reduced when cell proliferation is inhibited. As the neocortex matures, sustained expression of the oligodendrocyte lineage-specific key transcription factor Sox10 becomes less dependent on Olig2. Together, our findings suggest a gradual stabilization of the oligodendrocyte lineage genes and loss of lineage plasticity during the first 3 weeks after birth, possibly due to nuclear reorganization.
Keywords: NG2; Olig2; Sox10; astrocyte; lineage; oligodendrocyte.
Copyright © 2018 the authors 0270-6474/18/382359-13$15.00/0.
Figures
Similar articles
-
Olig2-dependent developmental fate switch of NG2 cells.Development. 2012 Jul;139(13):2299-307. doi: 10.1242/dev.078873. Epub 2012 May 23. Development. 2012. PMID: 22627280 Free PMC article.
-
Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum.Cell Death Dis. 2013 Mar 14;4(3):e546. doi: 10.1038/cddis.2013.74. Cell Death Dis. 2013. PMID: 23492777 Free PMC article.
-
Sulfatase 2 promotes generation of a spinal cord astrocyte subtype that stands out through the expression of Olig2.Glia. 2019 Aug;67(8):1478-1495. doi: 10.1002/glia.23621. Epub 2019 Apr 13. Glia. 2019. PMID: 30980466 Free PMC article.
-
Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells.J Neurocytol. 2002 Jul-Aug;31(6-7):437-55. doi: 10.1023/a:1025783412651. J Neurocytol. 2002. PMID: 14501215 Review.
-
Lineage, fate, and fate potential of NG2-glia.Brain Res. 2016 May 1;1638(Pt B):116-128. doi: 10.1016/j.brainres.2015.08.013. Epub 2015 Aug 21. Brain Res. 2016. PMID: 26301825 Free PMC article. Review.
Cited by
-
Olig2 Ablation in Immature Oligodendrocytes Does Not Enhance CNS Myelination and Remyelination.J Neurosci. 2022 Nov 9;42(45):8542-8555. doi: 10.1523/JNEUROSCI.0237-22.2022. Epub 2022 Oct 5. J Neurosci. 2022. PMID: 36198499 Free PMC article.
-
Direct reprogramming of oligodendrocyte precursor cells into GABAergic inhibitory neurons by a single homeodomain transcription factor Dlx2.Sci Rep. 2021 Feb 11;11(1):3552. doi: 10.1038/s41598-021-82931-9. Sci Rep. 2021. PMID: 33574458 Free PMC article.
-
Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease.Cells. 2021 Aug 10;10(8):2045. doi: 10.3390/cells10082045. Cells. 2021. PMID: 34440814 Free PMC article. Review.
-
Novel guanidine compounds inhibit platelet-derived growth factor receptor alpha transcription and oligodendrocyte precursor cell proliferation.Glia. 2021 Mar;69(3):792-811. doi: 10.1002/glia.23930. Epub 2020 Oct 24. Glia. 2021. PMID: 33098183 Free PMC article.
-
Massively parallel disruption of enhancers active in human neural stem cells.Cell Rep. 2024 Feb 27;43(2):113693. doi: 10.1016/j.celrep.2024.113693. Epub 2024 Jan 23. Cell Rep. 2024. PMID: 38271204 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
