Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression
- PMID: 29382712
- PMCID: PMC5830519
- DOI: 10.1523/JNEUROSCI.2568-17.2018
Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression
Abstract
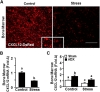
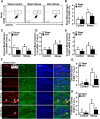
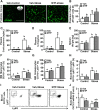
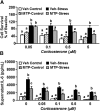
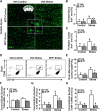
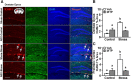
Repeated social defeat (RSD) stress promotes the release of bone marrow-derived monocytes into circulation that are recruited to the brain, where they augment neuroinflammation and cause prolonged anxiety-like behavior. Physiological stress activates the sympathetic nervous system and hypothalamic-pituitary-adrenal gland (HPA) axis, and both of these systems play a role in the physiological, immunological, and behavioral responses to stress. The purpose of this study was to delineate the role of HPA activation and corticosterone production in the immunological responses to stress in male C57BL/6 mice. Here, surgical (adrenalectomy) and pharmacological (metyrapone) interventions were used to abrogate corticosterone signaling during stress. We report that both adrenalectomy and metyrapone attenuated the stress-induced release of monocytes into circulation. Neither intervention altered the production of monocytes during stress, but both interventions enhanced retention of these cells in the bone marrow. Consistent with this observation, adrenalectomy and metyrapone also prevented the stress-induced reduction of a key retention factor, CXCL12, in the bone marrow. Corticosterone depletion with metyrapone also abrogated the stress-induced glucocorticoid resistance of myeloid cells. In the brain, these corticosterone-associated interventions attenuated stress-induced microglial remodeling, neurovascular expression of the adhesion molecule intercellular cell adhesion molecule-1, prevented monocyte accumulation and neuroinflammatory signaling. Overall, these results indicate that HPA activation and corticosterone production during repeated social defeat stress are critical for monocyte release into circulation, glucocorticoid resistance of myeloid cells, and enhanced neurovascular cell adhesion molecule expression.SIGNIFICANCE STATEMENT Recent studies of stress have identified the presence of monocytes that show an exaggerated inflammatory response to immune challenge and are resistant to the suppressive effects of glucocorticoids. Increased presence of these proinflammatory monocytes has been implicated in neuropsychiatric symptoms and the development of chronic cardiovascular, autoimmune, and metabolic disorders. In the current study, we show novel evidence that corticosterone produced during stress enhances the release of proinflammatory monocytes from the bone marrow into circulation, augments their recruitment to the brain and the induction of a neuroinflammatory profile. Overproduction of corticosterone during stress is also the direct cause of glucocorticoid resistance, a key phenotype in individuals exposed to chronic stress. Inhibiting excess corticosterone production attenuates these inflammatory responses to stress.
Keywords: HPA axis; corticosterone; inflammation; monocytes; repeated social defeat.
Copyright © 2018 the authors 0270-6474/18/382328-13$15.00/0.
Figures

Similar articles
-
Unique brain endothelial profiles activated by social stress promote cell adhesion, prostaglandin E2 signaling, hypothalamic-pituitary-adrenal axis modulation, and anxiety.Neuropsychopharmacology. 2022 Dec;47(13):2271-2282. doi: 10.1038/s41386-022-01434-x. Epub 2022 Sep 14. Neuropsychopharmacology. 2022. PMID: 36104533 Free PMC article.
-
Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress.Psychoneuroendocrinology. 2016 Jun;68:163-70. doi: 10.1016/j.psyneuen.2016.02.027. Epub 2016 Feb 26. Psychoneuroendocrinology. 2016. PMID: 26974501 Free PMC article.
-
Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety.Mol Psychiatry. 2018 Jun;23(6):1421-1431. doi: 10.1038/mp.2017.64. Epub 2017 Apr 4. Mol Psychiatry. 2018. PMID: 28373688 Free PMC article.
-
Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety.Neuroscience. 2015 Mar 19;289:429-42. doi: 10.1016/j.neuroscience.2015.01.001. Epub 2015 Jan 14. Neuroscience. 2015. PMID: 25596319 Free PMC article. Review.
-
Stress-Induced Microglia Activation and Monocyte Trafficking to the Brain Underlie the Development of Anxiety and Depression.Curr Top Behav Neurosci. 2017;31:155-172. doi: 10.1007/7854_2016_25. Curr Top Behav Neurosci. 2017. PMID: 27352390 Review.
Cited by
-
Neurobiology of resilience in depression: immune and vascular insights from human and animal studies.Eur J Neurosci. 2021 Jan;53(1):183-221. doi: 10.1111/ejn.14547. Epub 2019 Sep 13. Eur J Neurosci. 2021. PMID: 31421056 Free PMC article. Review.
-
Oligodendroglia Are Primed for Antigen Presentation in Response to Chronic Stress-Induced Microglial-Derived Inflammation.Glia. 2025 Jun;73(6):1130-1147. doi: 10.1002/glia.24661. Epub 2024 Dec 24. Glia. 2025. PMID: 39719686
-
Cannabinoid receptor 1 signalling modulates stress susceptibility and microglial responses to chronic social defeat stress.Transl Psychiatry. 2021 Mar 15;11(1):164. doi: 10.1038/s41398-021-01283-0. Transl Psychiatry. 2021. PMID: 33723234 Free PMC article.
-
Glucocorticoid resistance and β2-adrenergic receptor signaling pathways promote peripheral pro-inflammatory conditions associated with chronic psychological stress: A systematic review across species.Neurosci Biobehav Rev. 2021 Sep;128:117-135. doi: 10.1016/j.neubiorev.2021.06.013. Epub 2021 Jun 8. Neurosci Biobehav Rev. 2021. PMID: 34116126 Free PMC article.
-
Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats.Brain Behav Immun. 2020 Feb;84:180-199. doi: 10.1016/j.bbi.2019.11.023. Epub 2019 Nov 27. Brain Behav Immun. 2020. PMID: 31785394 Free PMC article.
References
-
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS (2011) Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208:261–271. 10.1084/jem.20101688 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
