Tyrosine phosphorylation switching of a G protein
- PMID: 29382719
- PMCID: PMC5880138
- DOI: 10.1074/jbc.RA117.000163
Tyrosine phosphorylation switching of a G protein
Abstract
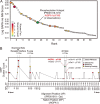
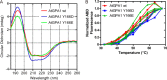
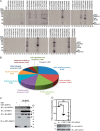
Heterotrimeric G protein complexes are molecular switches relaying extracellular signals sensed by G protein-coupled receptors (GPCRs) to downstream targets in the cytoplasm, which effect cellular responses. In the plant heterotrimeric GTPase cycle, GTP hydrolysis, rather than nucleotide exchange, is the rate-limiting reaction and is accelerated by a receptor-like regulator of G signaling (RGS) protein. We hypothesized that posttranslational modification of the Gα subunit in the G protein complex regulates the RGS-dependent GTPase cycle. Our structural analyses identified an invariant phosphorylated tyrosine residue (Tyr166 in the Arabidopsis Gα subunit AtGPA1) located in the intramolecular domain interface where nucleotide binding and hydrolysis occur. We also identified a receptor-like kinase that phosphorylates AtGPA1 in a Tyr166-dependent manner. Discrete molecular dynamics simulations predicted that phosphorylated Tyr166 forms a salt bridge in this interface and potentially affects the RGS protein-accelerated GTPase cycle. Using a Tyr166 phosphomimetic substitution, we found that the cognate RGS protein binds more tightly to the GDP-bound Gα substrate, consequently reducing its ability to accelerate GTPase activity. In conclusion, we propose that phosphorylation of Tyr166 in AtGPA1 changes the binding pattern with AtRGS1 and thereby attenuates the steady-state rate of the GTPase cycle. We coin this newly identified mechanism "substrate phosphoswitching."
Keywords: G protein-coupled receptor (GPCR); GTP hydrolysis; GTPase; GTPase-activating protein (GAP); guanine-nucleotide exchange factor; heterotrimeric G protein; phosphotyrosine signaling; protein phosphorylation; regulator of G protein signaling (RGS); substrate phosphoswitching.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
Functional reconstitution of an atypical G protein heterotrimer and regulator of G protein signaling protein (RGS1) from Arabidopsis thaliana.J Biol Chem. 2011 Apr 15;286(15):13143-50. doi: 10.1074/jbc.M110.190355. Epub 2011 Feb 16. J Biol Chem. 2011. PMID: 21325279 Free PMC article.
-
G protein activation without a GEF in the plant kingdom.PLoS Genet. 2012 Jun;8(6):e1002756. doi: 10.1371/journal.pgen.1002756. Epub 2012 Jun 28. PLoS Genet. 2012. PMID: 22761582 Free PMC article.
-
The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits.Int J Biol Sci. 2005;1(2):51-66. doi: 10.7150/ijbs.1.51. Epub 2005 Apr 1. Int J Biol Sci. 2005. PMID: 15951850 Free PMC article. Review.
-
GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling.Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17317-22. doi: 10.1073/pnas.0704751104. Epub 2007 Oct 19. Proc Natl Acad Sci U S A. 2007. PMID: 17951432 Free PMC article.
-
Purification and in vitro functional analysis of the Arabidopsis thaliana regulator of G-protein signaling-1.Methods Enzymol. 2004;389:320-38. doi: 10.1016/S0076-6879(04)89019-0. Methods Enzymol. 2004. PMID: 15313574 Review.
Cited by
-
G-Protein Phosphorylation: Aspects of Binding Specificity and Function in the Plant Kingdom.Int J Mol Sci. 2022 Jun 11;23(12):6544. doi: 10.3390/ijms23126544. Int J Mol Sci. 2022. PMID: 35742988 Free PMC article. Review.
-
Plant G-protein activation: connecting to plant receptor kinases.Cell Res. 2018 Jul;28(7):697-698. doi: 10.1038/s41422-018-0046-2. Cell Res. 2018. PMID: 29844582 Free PMC article. No abstract available.
-
A molecular dynamics study of membrane positioning for 7-transmembrane RGS proteins to modulate G-protein-mediated signaling in plants.Comput Struct Biotechnol J. 2025 Apr 11;27:1529-1537. doi: 10.1016/j.csbj.2025.04.013. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40270710 Free PMC article.
-
GCR1 Positively Regulates UV-B- and Ethylene-Induced Stomatal Closure via Activating GPA1-Dependent ROS and NO Production.Int J Mol Sci. 2022 May 15;23(10):5512. doi: 10.3390/ijms23105512. Int J Mol Sci. 2022. PMID: 35628324 Free PMC article.
-
Heterotrimeric G Proteins in Plants: Canonical and Atypical Gα Subunits.Int J Mol Sci. 2021 Oct 31;22(21):11841. doi: 10.3390/ijms222111841. Int J Mol Sci. 2021. PMID: 34769272 Free PMC article. Review.
References
-
- Johnston C. A., Taylor J. P., Gao Y., Kimple A. J., Grigston J. C., Chen J.-G., Siderovski D. P., Jones A. M., and Willard F. S. (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 17317–17322 10.1073/pnas.0704751104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

