The protonation states of GTP and GppNHp in Ras proteins
- PMID: 29382720
- PMCID: PMC5857994
- DOI: 10.1074/jbc.RA117.001110
The protonation states of GTP and GppNHp in Ras proteins
Abstract
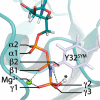
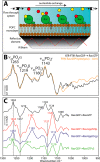
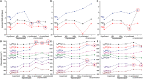
The small GTPase Ras transmits signals in a variety of cellular signaling pathways, most prominently in cell proliferation. GTP hydrolysis in the active center of Ras acts as a prototype for many GTPases and is the key to the understanding of several diseases, including cancer. Therefore, Ras has been the focus of intense research over the last decades. A recent neutron diffraction crystal structure of Ras indicated a protonated γ-guanylyl imidodiphosphate (γ-GppNHp) group, which has put the protonation state of GTP in question. A possible protonation of GTP was not considered in previously published mechanistic studies. To determine the detailed prehydrolysis state of Ras, we calculated infrared and NMR spectra from quantum mechanics/molecular mechanics (QM/MM) simulations and compared them with those from previous studies. Furthermore, we measured infrared spectra of GTP and several GTP analogs bound to lipidated Ras on a membrane system under near-native conditions. Our findings unify results from previous studies and indicate a structural model confirming the hypothesis that γ-GTP is fully deprotonated in the prehydrolysis state of Ras.
Keywords: Fourier transform IR, FTIR; GTP hydrolysis; GTPase; NMR; QM/MM simulation; Ras protein; molecular dynamics; nuclear magnetic resonance; quantum chemistry.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Kötting C., Güldenhaupt J., and Gerwert K. (2012) Time-resolved FTIR spectroscopy for monitoring protein dynamics exemplified by functional studies of Ras protein bound to a lipid bilayer. Chem. Phys. 396, 72–83 10.1016/j.chemphys.2011.08.007 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

