Dimerization of sortilin regulates its trafficking to extracellular vesicles
- PMID: 29382723
- PMCID: PMC5868269
- DOI: 10.1074/jbc.RA117.000732
Dimerization of sortilin regulates its trafficking to extracellular vesicles
Abstract
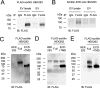
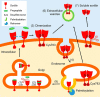
Extracellular vesicles (EVs) play a critical role in intercellular communication by transferring microRNAs, lipids, and proteins to neighboring cells. Sortilin, a sorting receptor that directs target proteins to the secretory or endocytic compartments of cells, is found in both EVs and cells. In many human diseases, including cancer and cardiovascular disorders, sortilin expression levels are atypically high. To elucidate the relationship between cardiovascular disease, particularly vascular calcification, and sortilin expression levels, we explored the trafficking of sortilin in both the intracellular and extracellular milieu. We previously demonstrated that sortilin promotes vascular calcification via its trafficking of tissue-nonspecific alkaline phosphatase to EVs. Although recent reports have noted that sortilin is regulated by multiple post-translational modifications, the precise mechanisms of sortilin trafficking still need to be determined. Here, we show that sortilin forms homodimers with an intermolecular disulfide bond at the cysteine 783 (Cys783) residue, and because Cys783 can be palmitoylated, it could be shared via palmitoylation and an intermolecular disulfide bond. Formation of this intermolecular disulfide bond leads to trafficking of sortilin to EVs by preventing palmitoylation, which further promotes sortilin trafficking to the Golgi apparatus. Moreover, we found that sortilin-derived propeptide decreased sortilin homodimers within EVs. In conclusion, sortilin is transported to EVs via the formation of homodimers with an intermolecular disulfide bond, which is endogenously regulated by its own propeptide. Therefore, we propose that inhibiting dimerization of sortilin acts as a new therapeutic strategy for the treatment of EV-associated diseases, including vascular calcification and cancer.
Keywords: calcification; dimerization; extracellular vesicles; intermolecular disulfide bond; sortilin; sorting; trafficking.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
S. I. and K. M. are employees of Kowa Company, Ltd
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

