Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience
- PMID: 29382821
- PMCID: PMC5802623
- DOI: 10.1038/s41398-017-0071-9
Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience
Abstract
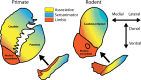
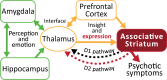
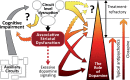
The stagnation in drug development for schizophrenia highlights the need for better translation between basic and clinical research. Understanding the neurobiology of schizophrenia presents substantial challenges but a key feature continues to be the involvement of subcortical dopaminergic dysfunction in those with psychotic symptoms. Our contemporary knowledge regarding dopamine dysfunction has clarified where and when dopaminergic alterations may present in schizophrenia. For example, clinical studies have shown patients with schizophrenia show increased presynaptic dopamine function in the associative striatum, rather than the limbic striatum as previously presumed. Furthermore, subjects deemed at high risk of developing schizophrenia show similar presynaptic dopamine abnormalities in the associative striatum. Thus, our view of subcortical dopamine function in schizophrenia continues to evolve as we accommodate this newly acquired information. However, basic research in animal models has been slow to incorporate these clinical findings. For example, psychostimulant-induced locomotion, the commonly utilised phenotype for positive symptoms in rodents, is heavily associated with dopaminergic activation in the limbic striatum. This anatomical misalignment has brought into question how we assess positive symptoms in animal models and represents an opportunity for improved translation between basic and clinical research. The current review focuses on the role of subcortical dopamine dysfunction in psychosis and schizophrenia. We present and discuss alternative phenotypes that may provide a more translational approach to assess the neurobiology of positive symptoms in schizophrenia. Incorporation of recent clinical findings is essential if we are to develop meaningful translational animal models.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Aymard N, et al. Long-term pharmacoclinical follow-up in schizophrenic patients treated with risperidone - Plasma and red blood cell concentrations of risperidone and its 9-hydroxymetabolite and their relationship to whole blood serotonin and tryptophan, plasma homovanillic acid, 5-hydroxyindoleacetic acid, dihydroxyphenylethyleneglycol and clinical evaluations. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:975–988. doi: 10.1016/S0278-5846(02)00218-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

