Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence
- PMID: 29382857
- PMCID: PMC5790005
- DOI: 10.1038/s41598-018-19767-3
Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence
Abstract
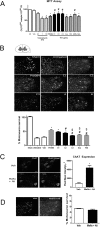
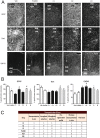
Here we used a systems biology approach and artificial intelligence to identify a neuroprotective agent for the treatment of peripheral nerve root avulsion. Based on accumulated knowledge of the neurodegenerative and neuroprotective processes that occur in motoneurons after root avulsion, we built up protein networks and converted them into mathematical models. Unbiased proteomic data from our preclinical models were used for machine learning algorithms and for restrictions to be imposed on mathematical solutions. Solutions allowed us to identify combinations of repurposed drugs as potential neuroprotective agents and we validated them in our preclinical models. The best one, NeuroHeal, neuroprotected motoneurons, exerted anti-inflammatory properties and promoted functional locomotor recovery. NeuroHeal endorsed the activation of Sirtuin 1, which was essential for its neuroprotective effect. These results support the value of network-centric approaches for drug discovery and demonstrate the efficacy of NeuroHeal as adjuvant treatment with surgical repair for nervous system trauma.
Conflict of interest statement
M.C. and R.V. are employees and J.M.M. is founder and C.E.O. of Anaxomics Biotech. J.M.M. contributed to the design of TPMS. The other authors D.R., J.F., M.H.G., T.L.R., E.G., F.R.P., X.N., V.P., A.B., and C.C. declare no competing interests. NeuroHeal is currently under patent review.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

