Modulation of Neurally Mediated Vasodepression and Bradycardia by Electroacupuncture through Opioids in Nucleus Tractus Solitarius
- PMID: 29382866
- PMCID: PMC5789879
- DOI: 10.1038/s41598-018-19672-9
Modulation of Neurally Mediated Vasodepression and Bradycardia by Electroacupuncture through Opioids in Nucleus Tractus Solitarius
Abstract
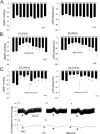
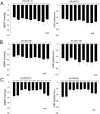
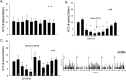
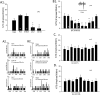
Stimulation of vagal afferent endings with intravenous phenylbiguanide (PBG) causes both bradycardia and vasodepression, simulating neurally mediated syncope. Activation of µ-opioid receptors in the nucleus tractus solitarius (NTS) increases blood pressure. Electroacupuncture (EA) stimulation of somatosensory nerves underneath acupoints P5-6, ST36-37, LI6-7 or G37-39 selectively but differentially modulates sympathoexcitatory responses. We therefore hypothesized that EA-stimulation at P5-6 or ST36-37, but not LI6-7 or G37-39 acupoints, inhibits the bradycardia and vasodepression through a µ-opioid receptor mechanism in the NTS. We observed that stimulation at acupoints P5-6 and ST36-37 overlying the deep somatosensory nerves and LI6-7 and G37-39 overlying cutaneous nerves differentially evoked NTS neural activity in anesthetized and ventilated animals. Thirty-min of EA-stimulation at P5-6 or ST36-37 reduced the depressor and bradycardia responses to PBG while EA at LI6-7 or G37-39 did not. Congruent with the hemodynamic responses, EA at P5-6 and ST36-37, but not at LI6-7 and G37-39, reduced vagally evoked activity of cardiovascular NTS cells. Finally, opioid receptor blockade in the NTS with naloxone or a specific μ-receptor antagonist reversed P5-6 EA-inhibition of the depressor, bradycardia and vagally evoked NTS activity. These data suggest that point specific EA stimulation inhibits PBG-induced vasodepression and bradycardia responses through a μ-opioid mechanism in the NTS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Neurogenic Hypotension and Bradycardia Modulated by Electroacupuncture in Hypothalamic Paraventricular Nucleus.Front Neurosci. 2022 Jul 22;16:934752. doi: 10.3389/fnins.2022.934752. eCollection 2022. Front Neurosci. 2022. PMID: 35958987 Free PMC article.
-
Modulation of cardiopulmonary depressor reflex in nucleus ambiguus by electroacupuncture: roles of opioids and γ-aminobutyric acid.Am J Physiol Regul Integr Comp Physiol. 2012 Apr;302(7):R833-44. doi: 10.1152/ajpregu.00440.2011. Epub 2011 Dec 28. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22204951 Free PMC article.
-
GABA in nucleus tractus solitarius participates in electroacupuncture modulation of cardiopulmonary bradycardia reflex.Am J Physiol Regul Integr Comp Physiol. 2014 Dec 1;307(11):R1313-23. doi: 10.1152/ajpregu.00300.2014. Epub 2014 Sep 17. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25231352 Free PMC article.
-
Mechanism of the inhibitory effect of electroacupuncture on experimental arrhythmias.J Acupunct Meridian Stud. 2013 Apr;6(2):69-81. doi: 10.1016/j.jams.2012.11.001. Epub 2012 Nov 28. J Acupunct Meridian Stud. 2013. PMID: 23591002 Review.
-
Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius.Prog Neurobiol. 1996 Jan;48(1):21-53. doi: 10.1016/0301-0082(95)00034-8. Prog Neurobiol. 1996. PMID: 8830347 Review.
Cited by
-
A Biophysical Model for Cardiovascular Effects of Acupuncture-Underlying Mechanisms Based on First Principles.Med Acupunct. 2022 Dec 1;34(6):353-370. doi: 10.1089/acu.2022.0050. Epub 2022 Dec 12. Med Acupunct. 2022. PMID: 36644426 Free PMC article. Review.
-
Pain Relief Dependent on IL-17-CD4+ T Cell-β-Endorphin Axis in Rat Model of Brachial Plexus Root Avulsion After Electroacupuncture Therapy.Front Neurosci. 2021 Feb 9;14:596780. doi: 10.3389/fnins.2020.596780. eCollection 2020. Front Neurosci. 2021. PMID: 33633527 Free PMC article.
-
Neurogenic Hypotension and Bradycardia Modulated by Electroacupuncture in Hypothalamic Paraventricular Nucleus.Front Neurosci. 2022 Jul 22;16:934752. doi: 10.3389/fnins.2022.934752. eCollection 2022. Front Neurosci. 2022. PMID: 35958987 Free PMC article.
-
A bibliometric analysis of 100 top-cited journal articles related to acupuncture regulation of the autonomic nervous system.Front Neurosci. 2022 Dec 22;16:1086087. doi: 10.3389/fnins.2022.1086087. eCollection 2022. Front Neurosci. 2022. PMID: 36620457 Free PMC article.
-
Acupuncture activates a direct pathway from the nucleus tractus solitarii to the rostral ventrolateral medulla.Brain Res. 2019 Apr 1;1708:69-77. doi: 10.1016/j.brainres.2018.12.009. Epub 2018 Dec 6. Brain Res. 2019. PMID: 30529283 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

