Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity
- PMID: 29382880
- PMCID: PMC5789897
- DOI: 10.1038/s41598-018-20305-4
Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity
Abstract
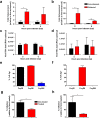
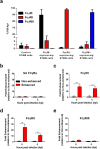
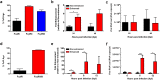
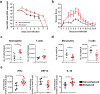
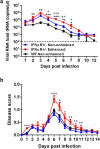
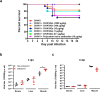
The arthropod-transmitted chikungunya virus (CHIKV) causes a flu-like disease that is characterized by incapacitating arthralgia. The re-emergence of CHIKV and the continual risk of new epidemics have reignited research in CHIKV pathogenesis. Virus-specific antibodies have been shown to control virus clearance, but antibodies present at sub-neutralizing concentrations can also augment virus infection that exacerbates disease severity. To explore this occurrence, CHIKV infection was investigated in the presence of CHIKV-specific antibodies in both primary human cells and a murine macrophage cell line, RAW264.7. Enhanced attachment of CHIKV to the primary human monocytes and B cells was observed while increased viral replication was detected in RAW264.7 cells. Blocking of specific Fc receptors (FcγRs) led to the abrogation of these observations. Furthermore, experimental infection in adult mice showed that animals had higher viral RNA loads and endured more severe joint inflammation in the presence of sub-neutralizing concentrations of CHIKV-specific antibodies. In addition, CHIKV infection in 11 days old mice under enhancing condition resulted in higher muscles viral RNA load detected and death. These observations provide the first evidence of antibody-mediated enhancement in CHIKV infection and pathogenesis and could also be relevant for other important arboviruses such as Zika virus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

