Contaminant-Activated Visible Light Photocatalysis
- PMID: 29382935
- PMCID: PMC5789862
- DOI: 10.1038/s41598-018-19972-0
Contaminant-Activated Visible Light Photocatalysis
Abstract
Pristine titanium dioxide (TiO2) absorbs ultraviolet light and reflects the entire visible spectrum. This optical response of TiO2 has found widespread application as white pigments in paper, paints, pharmaceuticals, foods and plastic industries; and as a UV absorber in cosmetics and photocatalysis. However, pristine TiO2 is considered to be inert under visible light for these applications. Here we show for the first time that a bacterial contaminant (Staphylococcus aureus-a MRSA surrogate) in contact with TiO2 activates its own photocatalytic degradation under visible light. The present study delineates the critical role of visible light absorption by contaminants and electronic interactions with anatase in photocatalytic degradation using two azo dyes (Mordant Orange and Procion Red) that are highly stable because of their aromaticity. An auxiliary light harvester, polyhydroxy fullerenes, was successfully used to accelerate photocatalytic degradation of contaminants. We designed a contaminant-activated, transparent, photocatalytic coating for common indoor surfaces and conducted a 12-month study that proved the efficacy of the coating in killing bacteria and holding bacterial concentrations generally below the benign threshold. Data collected in parallel with this study showed a substantial reduction in the incidence of infections.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

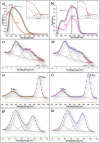
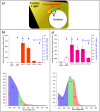



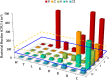
References
-
- Page K, Wilson M, Parkin IP. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. Journal of Materials Chemistry. 2009;19:3819–3831. doi: 10.1039/b818698g. - DOI
-
- Lederer JW, Jarvis WR, Thomas L, Ritter J. Multicenter cohort study to assess the impact of a silver-alloy and hydrogel-coated urinary catheter on symptomatic catheter-associated urinary tract infections. Journal of wound, ostomy, and continence nursing: official publication of The Wound, Ostomy and Continence Nurses Society / WOCN. 2014;41:473–480. doi: 10.1097/WON.0000000000000056. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

